
IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
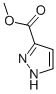
Product NameMETHYL 1H-PYRAZOLE-3-CARBOXYLATEIUPAC Namemethyl 1H-pyrazole-5-carboxylateMolecular StructureCAS Registry Number 462-08-8SynonymsMethyl 1H-pyrazole-3-carboxylate15366-34-4DTXSID10340565DTXCID30291646675-309-2methyl 1h-pyrazole-5-carboxylate1H-Pyrazole-3-carboxylic acid methyl estermethyl pyrazole-3-carboxylate394658-31-22H-Pyrazole-3-carboxylic acid methyl ester3-Methoxycarbonylpyrazole4-Pyrazolecarboxylic Acid Methyl EsterMFCD00649381MFCD04967319pyrazole-3-carboxylic acid methyl ester1H-Pyrazole-3-carboxylic acid, methyl ester5-methoxycarbonylpyrazolemethyl 3-pyrazolecarboxylateSCHEMBL560682methyl 1H-pyrazol-5-carboxylateALBB-003661BCP26620Methyl 1H-pyrazole-3-carboxylate #GEO-01919STK257454AKOS000305749AKOS000321526AC-8946BCP9000067CS-W008807PB10159METHYL 2H-PYRAZOLE-3-CARBOXYLATENCGC00330701-01AC-23119SY013591TS-01651DB-005734M2444Methyl 1H-pyrazole-3-carboxylate, AldrichCPREN300-128969H57052P10300AB01325199-02F0912-0219Z445222298Molecular FormulaC5H6N2O2Molecular Weight126.11InChIInChI=1S/C5H6N2/c6-5-2-1-3-7-4-5/h1-4H,6H2InChI KeyORUCTBNNYKZMSK-UHFFFAOYSA-NSMILESCOC(=O)C1=CC=NN1
Patent InformationPatent IDTitlePublication DateCN119330964Pyrrolopyridine heterocyclic compound, preparation method thereof and application of pyrrolopyridine heterocyclic compound in preparation of medicine for treating Alzheimer disease2025CN117447362Green synthesis method of azobenzene oxide compound2024WO2024/233767INHIBITORS OF CYCLIC GMP-AMP SYNTHASE AND USES THEREOF2024CN118994022Preparation method and non-classical antibacterial activity application of 1-methyl-4-phenyl ether derivative2024WO2023/288195CD38 MODULATORS AND METHODS OF USE THEREOF2023WO2023/285787SULFUR COMPOUNDS AND PROCESSES AND INTERMEDIATES USEFUL IN THE PREPARATION THEREOF2023CN115838338Method for preparing amide2023
Physical Data
AppearanceWhite powder
Melting Point, °C 60 - 6261.4263 - 6456.361 - 6460 - 6361
Boiling Point, °C251250 - 252
Density, g·cm-3Reference Temperature, °CMeasurement Temperature, °C1.144251.24-1901.24
Description (Association (MCS))Adsorption isotherm
Spectra
Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Frequency (NMR Spectroscopy), MHzChemical shifts, Spectrum13Cchloroform-d1151Chemical shifts, Spectrum1Hchloroform-d1599Chemical shifts, Spectrum1Hchloroform-d1Chemical shifts, Spectrum13Cchloroform-d1Chemical shifts, Spectrum13Cchloroform-d1Chemical shifts, Spectrum1Hchloroform-d1Chemical shifts, Spectrum13Cchloroform-d1
Description (IR Spectroscopy)Solvent (IR Spectroscopy)ATR (attenuated total reflectance), BandsBands, Spectrumpotassium bromideSpectrumBandsIntensity of IR bands, Bands, SpectrumBandspotassium bromide
Description (UV/VIS Spectroscopy)Solvent (UV/VIS Spectroscopy)Comment (UV/VIS Spectroscopy)Absorption Maxima (UV/VIS), nmExt./Abs. Coefficient, l·mol-1cm-1SpectrumSpectrumwaterSpectrumchloroformAbsorption maximaH2O, H2SO4Ratio of solvents: 66percent2585740Absorption maximaH2O, H2SO4Ratio of solvents: 66percent2585730Absorption maximaH2O233, 2928070, 2960Absorption maximaH2O, NaOHRatio of solvents: 0.1N232, 2908600, 3120
Route of Synthesis (ROS)
Route of Synthesis (ROS) of Methyl 1H-pyrazole-3-carboxylate CAS 15366-34-4
ConditionsYieldWith tert.-butylnitrite; trimethylsilylazide In acetonitrile at 0 - 20℃; for 2h;100%Stage #1: pyridin-3-ylamine With hydrogenchloride; sodium nitrite In water for 0.5h; Cooling with ice;Stage #2: With sodium azide In water at 20℃; for 2h; Cooling with ice;Experimental Procedure4.1.1 General procedure for the preparation of compounds 2a-eGeneral procedure: Aniline (931mg, 10mmol) was dissolved with HCl (6mol·L-1, 10mL) in an ice bath. NaNO2 (1035mg, 15mmol) dissolved in 25mL water was added dropwise. The reaction mixture was stirred for 30min. Sodium azide (2600mg, 40mmol) dissolved in 50mL water was added dropwise. After this addition, the system was stirred for another 2h at room temperature. Then, the mixture was extracted with ethyl acetate and the combined organic extracts were washed with H2O, dried over anhydrous Na2SO4, filtered, and concentrated in vacuo to give 2a (1190mg, 100%) as yellow oil. The crude product was used directly without purification. Compounds 2b, 2c, 2d, 2e were obtained with 1b, 1c, 1d, 1e respectively by using the same method that described above.95%Stage #1: pyridin-3-ylamine With sulfuric acid; sodium nitrite In water at 0 - 55℃; for 0.416667h;Stage #2: With urea In water for 0.333333h;Stage #3: With sodium azide In water at 20℃; for 1.5h;Experimental Procedure1.2 3-(1H-1,2,3-Triazol-4-yl)pyridine (2)To a solution of 3.6 mL of H2SO4 98% wt and 21.0 mL of water heated at 55 °C, 3-aminopyridine (1.89 g, 20.10 mmol, 1 eq) was added.After 5 min, the solution was cooled at 0 °C and a solution of sodium nitrite (1.66 g, 2.41 mmol, 1.2 eq in 14.0 mL of water) was added dropwise.After 20 min, urea was added (0.24 g, 4.00 mmol, 0.2 eq) and after 20 min a solution of NaN3 (1.65 g, 24.00 mmol, 1.2 eq in 15 mL of water) was added dropwise.The reaction was then stirred at room temperature for 1.5 h and then quenched with saturated aqueous NaHCO3 and extracted with diethyl ether (*3).Evaporation of the solvent gave a brown oil which was enough pure to be used for the next step without purification (95%).1H NMR (300 MHz, CDCl3) δ 8.23-8.19 (m, 2-H), 7.17-7.12 (m, 2-H); 13C NMR (75 MHz, CDCl3) δ 145.9, 141.2, 137.0, 125.8, 124.1.95%
Safety and Hazards
Pictogram(s)SignalWarningGHS Hazard StatementsH315 (100%): Causes skin irritation H319 (100%): Causes serious eye irritation H335 (80%): May cause respiratory irritation Precautionary Statement CodesP261, P264, P264+P265, P271, P280, P302+P352, P304+P340, P305+P351+P338, P319, P321, P332+P317, P337+P317, P362+P364, P403+P233, P405, and P501(The corresponding statement to each P-code can be found at the GHS Classification page.)
Other Data
TransportationUnder the room temperature and away from lightHS CodeStorageUnder the room temperature and away from lightShelf Life2 years
DruglikenessLipinski rules componentMolecular Weight94.116logP-0.047HBA2HBD1Matching Lipinski Rules4Veber rules componentPolar Surface Area (PSA)38.91Rotatable Bond (RotB)0Matching Veber Rules2 https://www.chemwhat.com/methyl-1h-pyrazole-3-carboxylate-cas-15366-34-4/
Comments
Post a Comment