
IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
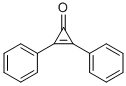
Product NameDIPHENYLCYCLOPROPENONEIUPAC Name2,3-diphenylcycloprop-2-en-1-oneMolecular StructureCAS Registry Number 886-38-4EINECS Number212-948-4MDL NumberMFCD00001311Beilstein Registry Number608049Synonyms3-Pyridinamin;3-Pyridinamine;3-Pyridinamine;pyridin-3-amine;T6NJ CZ;3- Aminopyridine;3-Amino-pyridine;3-pyridylamine;Amino-3 pyridine;m-Aminopyridine;MS/MS-1064463;Pyridin-3-ylamine;Pyridine, 3-amino-;β-Aminopyridine462-08-8Molecular FormulaC15H10O Molecular Weight206.24InChIInChI=1S/C15H10O/c16-15-13(11-7-3-1-4-8-11)14(15)12-9-5-2-6-10-12/h1-10HInChI KeyHCIBTBXNLVOFER-UHFFFAOYSA-NSMILESC1=CC=C(C=C1)C2=C(C2=O)C3=CC=CC=C3
Patent InformationPatent IDTitlePublication DateCN114031497Ring-opening dichlorination reaction method of cyclopropenone and oxygen heterocyclic compound2022CN113929651Method for synthesizing alpha-pyrone compound2022CN113896630Ring-opening diiodination reaction method of cyclopropenone and oxygen heterocyclic compound2022 CN114573449Catalyst for preparing carboxylic acid acyl chloride through phosgenation reaction of carboxylic acid and application of catalyst2022CN112174880Preparation method of 1,3,4,6-tetra-substituted pyridinone derivative2022WO2022/188082METHOD FOR CARRYING OUT REACTION OF ISATIN COMPOUND AND CYCLOPROPENONE COMPOUND AT LOW CATALYTIC AMOUNT2022WO2022/226855METHOD FOR PREPARING PYRANO-INDOLE-2-ONE WITHOUT CATALYST2022
Physical Data
AppearanceOff-white to light cream fine powder
Melting Point, °C Solvent (Melting Point) 120 - 121119 - 120114 - 116121.587118 - 120cyclohexane120aq. ethanol
Density, g·cm-31.1
Description (Association (MCS))Solvent (Association (MCS))Temperature (Association (MCS)), °CPartner (Association (MCS))NMR spectrum of the complexD2O, acetone-d6201-ethoxy-2,3-diphenylcyclopropenium ion, 2.0 M DClO4, 1.5 M LiClO4Stability constant of the complex with ...Enthalpy of associationSpectrum of the complexFurther physical properties of the complex
Spectra
Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Temperature (NMR Spectroscopy), °C Frequency (NMR Spectroscopy), MHzChemical shifts, Spectrum1Hchloroform-d1Chemical shifts1Hchloroform-d1400Chemical shifts13Cchloroform-d1100Chemical shifts, Spectrum1Hchloroform-d1500Chemical shifts, Spectrum13Cchloroform-d1125Spectrum13Cchloroform-d1Chemical shifts, Spectrum1Hchloroform-d125500
Diphenylcyclopropenone CAS#: 886-38-4 NMR
Description (IR Spectroscopy)Solvent (IR Spectroscopy)Comment (IR Spectroscopy)Bandspotassium bromideATR-FTIR (attenuated total reflectance Fourier transform infrared spectroscopy), BandsBands, SpectrumdichloromethaneSpectrumacetonitrileSpectrumCCl4SpectrumhexaneBands1850 cm**(-1)
Description (UV/VIS Spectroscopy)Solvent (UV/VIS Spectroscopy)Comment (UV/VIS Spectroscopy)Absorption Maxima (UV/VIS), nmSpectrumH2aq. phosphate bufferSpectrumdimethyl sulfoxide302345, 325SpectrumcyclohexaneSpectrummethanolRemark: 25 deg Cmethanol295CH2Cl2226, 295
Route of Synthesis (ROS)
Route of Synthesis (ROS) of Diphenylcyclopropenone CAS 886-38-4
ConditionsYieldStage #1: 1,2,3,3-tetrachlorocyclopropene; benzene With aluminum (III) chloride In dichloromethane at -78 - 20℃; for 4h; Inert atmosphere; Sealed tube;Stage #2: With water In dichloromethane Inert atmosphere; Sealed tube;85%Stage #1: 1,2,3,3-tetrachlorocyclopropene; benzene With aluminum (III) chloride In dichloromethane for 0.5h;Stage #2: With water; sodium hydroxide In dichloromethane at 20℃; for 1.5h;Experimental Procedure4.2. General procedure for the synthesis of cyclopropenonesGeneral procedure: The AlCl 3 (0.67 g, 5 mmol) was added by portions to a solu- tion of one of the compounds 2a-d (25 mmol) and tetrachloro- cyclopropene (12.5 mmol) in anhydrous CH 2 Cl 2 (200 mL) un- der continuous stirring for 30 min. The stirring was continued for another 90 min at room temperature, then the mixture was poured in cold water (200 mL). The organic layer was sepa- rated, washed with water (2 ×50 mL) and dried with MgSO 4 . After the solvent was distilled offin vacuo , the residue was chromatographed on Al 2 O 3 using a hexane-CH 2 Cl 2 (3:1) mix- ture as eluent to afford compounds 2,3-diphenylcyclopropenone 3a , yield, 1.64 g, 7.8 mmol (63%), m.p. 120-121 °C (lit.: m.p. 121-121.5 °C ), 2,3- bis (4-methoxyphenyl)cyclopropenone 3b , yield, 2.23 g, 8.4 mmol (67%), m.p. 153-154 °C (lit.: m.p.153-155 °C ), 2,3-diferrocenyl-cyclopropenone 3c , yield, 4.5 g, 10.6 mmol (85.3%), m.p. 182-184 °C (lit.: m.p. 182-183 °C ), 2,3-diruthenocenylcyclopropenone 3d , yield, 3.8 g, 7.5 mmol (60%), m.p. 256-258 °C (lit.: m.p. 256-257 °C63%With aluminium trichloride; water 1.) 1,2-dichloroethane, room temp.;Stage #1: 1,2,3,3-tetrachlorocyclopropene; benzene With aluminum (III) chloride In carbon disulfide; dichloromethane at -78 - 20℃; Inert atmosphere;Stage #2: With water In carbon disulfide; dichloromethane Inert atmosphere;Experimental Procedure2,3-Diphenylcyclopropenone (8b) .In a 100 mL one-necked round-bottomed flask fitted with a septum, 1.6 mmol (0.285 g) of tetrachlorocyclopropene and 3.38 mmol (0.45 g) of aluminum chloride (AlCl3) were placed and 25 mL of dichloromethane (freshly dried over NaH) were added. The flask was cooled in an acetone/dry ice bath to 78 °C and stirred with a magnetic stirrer under argon atmosphere. A mixture of 3.2 mmol (0.25 g) of benzene and 2.5 mL of dry CH2Cl2 was added dropwise to the reaction mixture. After the benzene solution was completely dropped in, the mixture was magnetically stirred at 78 °C for 2 h and then allowed to warm to room temperature. The mixture was further stirred for 24 h and after that time, a portion of water was added, and the resulting solution was extracted twice with CH2Cl2. The organic layer was separated and washed with water and brine. The solvent was evaporated and the crude product, after recording the 1H NMR spectrum, was purified on a SiO2 column using a mixture of petroleum ether and ethyl acetate (ratio 4:1) as the eluent. The major fraction containing crude 8b was separated in 74% yield as pale-brown crystals. The product was not purified but used directly as obtained for the synthesis of thioketone 6b; therefore, the calculated yields of both 8b and 6b are unreliable. Pale-brown crystals; m.p. 119-121 °C (after washing with PE) (ref. , m.p. 120-121 °C). 1H NMR (600 MHz, CDCl3): d 7.70-7.73 (m, 6CHarom); 7.79-8.05 (m, 4CHarom).Stage #1: 1,2,3,3-tetrachlorocyclopropene; benzene With aluminum (III) chloride In dichloromethane Friedel-Crafts Alkylation;Stage #2: With sodium hydroxide In water Friedel-Crafts Alkylation;
Safety and Hazards
Pictogram(s)SignalWarningGHS Hazard StatementsH315 (85.7%): Causes skin irritation H317 (98%): May cause an allergic skin reaction Precautionary Statement CodesP261, P264, P272, P280, P302+P352, P321, P332+P317, P333+P317, P362+P364, and P501(The corresponding statement to each P-code can be found at the GHS Classification page.)
Other Data
TransportationUnder room temperature away from lightHS CodeStorageUnder room temperature away from lightShelf Life1 year
DruglikenessLipinski rules componentMolecular Weight206.244logP3.602HBA1HBD0Matching Lipinski Rules4Veber rules componentPolar Surface Area (PSA)17.07Rotatable Bond (RotB)2Matching Veber Rules2
Use PatternDIPHENYLCYCLOPROPENONE CAS#: 886-38-4 increasing peripheral blood mononuclear cell (PBMC) expression of interferon gamma in response to a viral or fungal immune stimulus in a person, infection of the skin or mucous membrane or causes lesions on the skin or mucous membrane,and increasing PBMC interferon gamma expression in response to a viral or fungal immune stimulus. https://www.chemwhat.com/diphenylcyclopropenone-cas-886-38-4/
Comments
Post a Comment