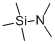
IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
Product NameN,N-DimethyltrimethylsilylamineIUPAC NameN-methyl-N-trimethylsilylmethanamineMolecular StructureCAS Registry Number 2083-91-2EINECS Number218-222-3MDL NumberMFCD00008297SynonymsN,N-Dimethyltrimethylsilylamine2083-91-2N,N-DimethylaminotrimethylsilaneN-(Trimethylsilyl)dimethylaminePentamethylsilylamineSilanamine, pentamethyl-(Dimethylamino)trimethylsilaneSilanamine, N,N,1,1,1-pentamethyl-N-methyl-N-trimethylsilylmethanamineTMSDMADimethylaminotrimethylsilane(n,n-dimethylamino)trimethylsilaneN,N,1,1,1-pentamethylsilanamineN,N-DimethyltrimethylsilamineDimethylamine, TMS derivativeR5D6N5EJ26MFCD00008297TMS-DMAtrimethylsilyldimethylamineDimethyl(trimethylsilyl)amineUNII-R5D6N5EJ26Dimethylamino(trimethyl)silaneN-Trimethylsilyl-dimethylamineSCHEMBL377532Dimethyl-trimethylsilanyl-amine(CH3)3SiN(CH3)2n,n-dimethyl-trimethylsilylamineDTXSID7062168EINECS 218-222-3EINECS 242-020-4CD5400n,n-dimethyl-n-(trimethylsilyl)amineN,N-Dimethyl-1-(trimethylsilyl)amineN,N-Dimethyltrimethylsilylamine, 97%AKOS006222984FS-5233N,N-DIMETHYL(TRIMETHYLSILYL)AMINEMethanamine, N-methyl-1-(trimethylsilyl)-NS00046579T0591S06925N,N-Dimethyltrimethylsilylamine, Selectophore(TM)J-013669N,N-Dimethyltrimethylsilylamine, purum, >=95.0% (GC)Molecular FormulaC5H15NSiMolecular Weight117.26InChIInChI=1S/C5H15NSi/c1-6(2)7(3,4)5/h1-5H3InChI KeyKAHVZNKZQFSBFW-UHFFFAOYSA-N Isomeric SMILESCN(C)(C)(C)C
Patent InformationPatent IDTitlePublication DateCN116854727Preparation method and device system of dialkylamino metal halide and imino tri (dialkylamino) metal complex2023US2020/248309Deposition Of Carbon Doped Silicon Oxide2020US2020/377445IMINE-TYPE QUATERNARY AMMONIUM SALT CATALYST, PREPARATION METHOD THEREOF AND USE THEREOF FOR PREPARATION OF POLYISOCYANATE COMPOSITION2020
Physical Data
AppearanceColorless to Light yellow Liquid
Boiling Point, °CPressure (Boiling Point), Torr84 - 87760.05182 - 848680 - 112116 - 11882 - 8677 - 79720
Density, g·cm-3Measurement Temperature, °C0.9810.746620
Spectra
Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Temperature (NMR Spectroscopy), °C Chemical shifts13Cbenzene-d6Chemical shifts1Hbenzene-d6Spectrum29Sibenzene, benzene-d6Chemical shifts13CCDCl30Chemical shifts13CCH2Cl2
Route of Synthesis (ROS)
Route of Synthesis (ROS) of N,N-Dimethyltrimethylsilylamine CAS 2083-91-2
ConditionsYieldIn methylbutane Ambient temperature;75%In octane; acetonitrile at 20℃; for 5h; Solvent; Reflux;Experimental ProcedureInto a 2 L 5-neck flask, 300 g of n-octane and 210 g of acetonitrile were charged. An oil bath was heated to 120° C. while stirring the resulting mixture into a reflux state, and then 64 g of acetonitrile containing a large amount of water accumulated in a reflux head was removed, and the resulting content was cooled. When an internal temperature was decreased to room temperature, 325 g of trimethylchlorosilane was charged into the 5-neck flask. Dimethylamine was fed from a gas phase part of the flask thereinto at a rate of 560 mL per minute for 4 hours at room temperature. A temperature of a reaction liquid was gradually increased up to 55° C. by an exothermic reaction. When loss of trimethylchlorosilane in a raw material was confirmed by a decrease in the temperature of the reaction liquid and gas chromatography (GC), feed of dimethylamine was stopped. A temperature of the oil bath was set to 80° C., and the resulting material was refluxed and aged for 1 hour. After cooling, 1040 g of the reaction liquid was obtained. The reaction liquid was filtered by a pressure filter, and a residue was washed with 50 g of acetonitrile to obtain 754 g of a filtrate. The filtrate was separated into a n-octane layer and an acetonitrile layer, and therefore was separated by a separating funnel to obtain 617 g of n-octane layer containing dimethylaminotrimethylsilane. When a GC analysis was conducted, 280 g of dimethylaminotrimethylsilane was contained therein and a reaction yield was 80%. Further, when hydrolyzable chlorine was measured, a content was 4 ppm. As a dehalogenation (chlorination) agent, 16 mg of potassium tert-butoxide, twice as many as moles of a chlorine component, was added to the n-octane layer, and a rectifying column prepared by packing HELI PACK into a column having a diameter of 2.5 cm and a length of 1 m was used at ordinary pressure to obtain 196 g of dimethylaminotrimethylsilane having a purity of 99% or more and a hydrolyzable chlorine component less than 1 ppm with a distillation yield of 70%.70%
Safety and Hazards
Pictogram(s)SignalDangerGHS Hazard StatementsH225 (100%): Highly Flammable liquid and vapor H260 (15.83%): In contact with water releases flammable gases which may ignite spontaneously H314 (55.4%): Causes severe skin burns and eye damage Precautionary Statement CodesP210, P223, P231+P232, P233, P240, P241, P242, P243, P260, P264, P280, P301+P330+P331, P302+P335+P334, P302+P361+P354, P303+P361+P353, P304+P340, P305+P354+P338, P316, P321, P363, P370+P378, P402+P404, P403+P235, P405, and P501(The corresponding statement to each P-code can be found at the GHS Classification page.)
Other Data
DruglikenessLipinski rules componentMolecular Weight117.266logP1.679HBA1HBD0Matching Lipinski Rules4Veber rules componentPolar Surface Area (PSA)3.24Rotatable Bond (RotB)1Matching Veber Rules2
Use PatternN,N-Dimethyltrimethylsilylamine CAS#: 2083-91-2 is an efficient deamination agent for methylsilanes, passivating agents, and reaction mixtures, which can be used in the coating of semiconductor materials.
https://www.chemwhat.com/nn-dimethyltrimethylsilylamine-cas-2083-91-2/
Comments
Post a Comment