IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
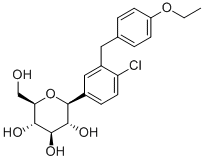
Product NameDapagliflozinIUPAC Name(2S,3R,4R,5S,6R)-2-phenyl]-6-(hydroxymethyl)oxane-3,4,5-triol Molecular StructureCAS Registry Number 461432-26-8EINECS Number639-683-0MDL NumberMFCMFCD13182359SynonymsDapagliflozin461432-26-8BMS-512148ForxigaBMS 512148(2S,3R,4R,5S,6R)-2-(4-chloro-3-(4-ethoxybenzyl)phenyl)-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triolDagagflozinUNII-1ULL0QJ8UC1ULL0QJ8UC(1S)-1,5-Anhydro-1-C-phenyl]-D-glucitolCHEBI:85078LYN-045(2S,3R,4R,5S,6R)-2-{4-chloro-3-phenyl}-6-(hydroxymethyl)oxane-3,4,5-triolCHEMBL429910DTXSID20905104Dapagliflozin QTERNMET COMPONENT DAPAGLIFOZINDAPAGLIFLOZIN COMPONENT QTERNMET(2S,3R,4R,5S,6R)-2-(4-Chloro-3-(4-ethoxybenzyl)phenyl)-6- (hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triolQTERNMET XR COMPONENT DAPAGLIFLOZINDAPAGLIFLOZIN COMPONENT OF QTERNMET XRForxiga (TN)D-Glucitol, 1,5-anhydro-1-C-(4-chloro-3-((4-ethoxyphenyl)methyl)phenyl)-, (1S)-CHEMBL3125458BMS512148(2S,3R,4R,5S,6R)-2-phenyl]-6-(hydroxymethyl)oxane-3,4,5-triol(2S,3R,4R,5S,6R)-2--6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triolDapagliflozin dapagliflozinedapagliflozinumDapagliflozina(1S)-1,5-anhydro-1-(4-chloro-3-(4-ethoxybenzyl)phenyl)-D-glucitol(1S)-1,5-anhydro-1--D-glucitol(2S,3R,4R,5S,6R)-2-phenyl]-6-(hydroxymethyl)oxane-3,4,5-triolDAPAGLIFLOZIN?S1548_SelleckC-aryl glucoside, 6DAPAGLIFLOZIN DAPAGLIFLOZIN Dapagliflozin (USAN/INN)2-(3-(4-Ethoxybenzyl)-4-chlorophenyl)-6-hydroxymethyltetrahydro-2H-pyran-3,4,5-triolDAPAGLIFLOZIN SCHEMBL157820DAPAGLIFLOZIN GTPL4594BDBM20880A10BK01EX-A005JVHXJTBJCFBINQ-ADAARDCZSA-NBCPP000265DTXCID101334207DAPAGLIFLOZIN AMY18541BDBM50448923MFCD13182359s1548AKOS005145763BCP9000583BL-0052BMS5121458CCG-229917CS-0781DB06292EX-7214NCGC00250402-09AC-24699BD164346HY-10450NS00099173A25150C22193D08897EN300-7407160Q409898J-500392BRD-K58160573-001-01-3BRD-K58160573-001-05-4Z2235801883(1S)-1,5-Anhydro-1-C-phenyl]-D-glucitol; (2S,3R,4R,5S,6R)-2-(4-Chloro-3-(4-ethoxybenzyl)phenyl)-6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol; (2S,3R,4R,5S,6R)-2--6-(hydroxymethyl)tetrahydro-2H-pyran-3,4,5-triol; BMS 512148; Dapagliflozin; Farxiga(2S, 3R, 4R, 5S, 6R)-2--6-hydroxymethyl-tetrahydro-pyran-3,4,5-triol(2S,3R,4R,5S,6R)-2-(3-(4-ethoxybenzyl)-4-chlorophenyl)-6-(hydroxymethyl)-tetrahydro-2H-pyran-3,4,5-triol(2S,3R,4R,5S,6R)-2-(3-(4-ethoxybenzyl)-4-chlorophenyl)-6-(hydroxymethyl)-tetrahydro-2H-pyran-3,4,5-triol;BMS-512148(2S,3R,4R,5S,6R)-2--6-hydroxymethyl-tetrahydro-pyran-3,4,5-triolLE6Molecular FormulaC21H25ClO6Molecular Weight408.9InChIInChI=1S/C21H25ClO6/c1-2-27-15-6-3-12(4-7-15)9-14-10-13(5-8-16(14)22)21-20(26)19(25)18(24)17(11-23)28-21/h3-8,10,17-21,23-26H,2,9,11H2,1H3/t17-,18-,19+,20-,21+/m1/s1InChI KeyCUYKNJBYIJFRCU-UHFFFAOYSA-NCanonical SMILESC1=CC(=CN=C1)N
Patent InformationPatent IDTitlePublication DateCN115785045Photocatalytic Endagliflozin precursor and synthesis method thereof2023WO2021/105152USE OF SGLT-2 INHIBITORS IN THE DRYING-OFF OF NON-HUMAN MAMMALS2021CN108285439Carbon glucoside sodium glucose transport protein body 2 inhibitor2018CN1086759766-halogenated glucose C-glycoside as well as preparation method and application thereof2018
Physical Data
Appearancewhite to grayish white powder
Melting Point, °C Solvent (Melting Point) 87 - 8988.3ethyl acetate, n-heptane88 - 8955.08 - 58.63
Description (Association (MCS))Temperature (Association (MCS)), °CPartner (Association (MCS))Association with compound21.84bovine serum albumin
Spectra
Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Temperature (NMR Spectroscopy), °C Frequency (NMR Spectroscopy), MHzChemical shifts, Spectrum1HChemical shifts, Spectrum13CChemical shifts, Spectrum1H25Chemical shifts, Spectrum13C25Chemical shifts, Spectrum1HChemical shifts1Hacetonitrile400
Description (IR Spectroscopy)Solvent (IR Spectroscopy)Bandssodium chlorideBands, Spectrumpotassium bromideBandsmethanolBands, Spectrum
Route of Synthesis (ROS)
Route of Synthesis (ROS) of Dapagliflozin CAS 461432-26-8
ConditionsYieldWith triethylsilane; boron trifluoride diethyl etherate In methanol; dichloromethane; ethyl acetate at -20 - 20℃; for 5h;Experimental ProcedureCompound 4a' (10.0 g, 22.1 mmol, 1.0 eq, purity 90.2%) was dissolved in dichloromethane (50 mL) and EtOAc (50 mL).The above solution was added to the reaction flask, and triethylsilane (7.7 g, 66.2 mmol, 3.0 eq) was added under nitrogen atmosphere, and the temperature was lowered to -20 ° C to -10 ° C, and boron trifluoride etherate (6.3 g, 44.2) was added dropwise. Methanol, 2.0 eq), after completion of the dropwise addition, the mixture was stirred for 4 hours, and then further stirred at room temperature for 1 hour. Saturated sodium hydrogen carbonate solution (100 mL) and ethyl acetate (100 mL) were added to the reaction mixture, and the mixture was separated, and the aqueous phase was extracted again with ethyl acetate (50 mL). The organic phase was combined and washed with water (50 mL) The aqueous sodium sulfate was dried, filtered and concentrated to dryness to give 7.4 g of the compound of formula I, yield 83.1%, purity 85.0%.83.1%Stage #1: (3R,4S,5S,6R)-2-(4-chloro-3-(4-ethoxybenzyl)phenyl)-6-(hydroxymethyl)-2-methoxytetrahydro-2H-pyran-3,4,5-triol With triethylsilane; boron trifluoride diethyl etherate In dichloromethane; acetonitrile at -10 - 20℃;Stage #2: With water; sodium hydrogencarbonate In dichloromethane; acetonitrileExperimental Procedure(2S,3R,4R,5S,6R)-2-(3-(4-Ethoxybenzyl)-4-chlorophenyl)-6- (hydroxymethyl)-tetrahydro-2H-pyran-3A5-triol: At about -10 0C, triethylsilane (343 mg, 2.96 mmol, 3.00 equiv) and boron trifluoride diethyl etherate (420 mg, 2.96 mmol, 3.00 equiv) were added dropwise to a stirred solution of (3R,4S,5S,6R)-2-(3-(4- ethoxybenzyl)-4-chlorophenyl)-6-(hydroxymethyl)-2-methoxy-tetrahydro-2H-pyran- 3,4,5-triol (300 mg, 0.68 mmol, 1.00 equiv) and acetonitrile / dichloromethane (50 niL). After stirring at ambient temperature for about 16 hours, the reaction was quenched by adding a saturated solution of sodium bicarbonate (50 mL). Standard extractive workup with ethyl acetate (3 x 200 mL) gave a crude residue which was first purified by silica gel column chromatography (eluted with dichloromethane / methanol (20 : 1) and then purified by preparative ΗPLC to remove the (2R,3R,4R,5S,6R)-2-(3-(4- ethoxybenzyl)-4-chlorophenyl)-6-(hydroxymethyl)-tetrahydro-2H-pyran-3,4,5-triol isomer. The title product was isolated as a white solid (130 mg; yield = 44%). 1H NMR (300 MHz, CDC13), δ: 7.37 (d, J = 8.1 Hz, IH), 7.32 (d, J = 1.8 Hz, IH), 7.24 (dd, J= 8.1, 1.8 Hz, IH), 7.09 (d, J= 8.4 Hz, IH), 6.82 (d, J= 8.4 Hz, IH), 4.83-5.00 (br m, 3H), 4.44 (s, IH), 3.93-4.03 (m, 5H), 3.67-3.71 (m, IH), 3.10-3.47 (m, 6H), 1.30 (t, J= 7.2 Hz, 3H); LC-MS : m/z= 453 (M+HCOO) ".44%
Safety and Hazards
Pictogram(s)SignalDangerGHS Hazard StatementsH302 (100%): Harmful if swallowed H318 (28.57%): Causes serious eye damage H319 (14.29%): Causes serious eye irritation H372 (57.14%): Causes damage to organs through prolonged or repeated exposure H373 (28.57%): May causes damage to organs through prolonged or repeated exposure H411 (14.29%): Toxic to aquatic life with long lasting effects H413 (28.57%): May cause long lasting harmful effects to aquatic life Precautionary Statement CodesP260, P264, P264+P265, P270, P273, P280, P301+P317, P305+P351+P338, P305+P354+P338, P317, P319, P330, P337+P317, P391, and P501(The corresponding statement to each P-code can be found at the GHS Classification page.)
Other Data
No data available
DruglikenessLipinski rules componentMolecular Weight408.879logP2.937HBA5HBD4Matching Lipinski Rules4Veber rules componentPolar Surface Area (PSA)99.38Rotatable Bond (RotB)6Matching Veber Rules2
Use PatternDapagliflozin is a sodium-glucose co-transporter 2 inhibitor (SGLT2i) and a new type of oral hypoglycemic drug. It can inhibit SGLT2 activity, inhibit proximal renal tubular sodium-glucose reabsorption, promote urinary glucose excretion, and thereby reduce blood glucose concentration.
https://www.chemwhat.com/dapagliflozin-cas-461432-26-8/
Comments
Post a Comment