IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
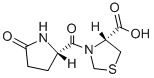
Product NamePidotimodIUPAC Name(4R)-3--1,3-thiazolidine-4-carboxylic acid Molecular StructureCAS Registry Number 121808-62-6MDL NumberMFCD00867583SynonymsPidotimod121808-62-6Pidotomod(R)-3-((S)-5-Oxopyrrolidine-2-carbonyl)thiazolidine-4-carboxylic acidPidotimod PGT/1APilimodNSC-759841(R)-3-((S)-5-Oxoprolyl)-4-thiazolidinecarboxylic acidDTXSID0046199Pidotimod (INN)(4R)-3--1,3-thiazolidine-4-carboxylic acid(4R)-3-{carbonyl}-1,3-thiazolidine-4-carboxylic acidNCGC00160516-01Polimod785363R681PidotimodumPigitilPidotimodum 4-Thiazolidinecarboxylic acid, 3-carbonyl]-, (4R)-SMR000466390CCRIS 7271BRN 6636310OnakaThymodolic acidAxilTimodolic acidPilimod (TN)Pidotimod - Bio-XUNII-785363R681PIDOTIMOD PIDOTIMOD (R-(R,S))-3-((5-Oxo-2-pyrrolidinyl)carbonyl)-4-thiazolidinecarboxylic acidPIDOTIMOD (4R)-3-CARBONYL]-4-THIAZOLIDINECARBOXYLIC ACID(R)-3-((S)-(5-Oxo-2-pyrrolidinyl)carbonyl)-thiazolidin-4-carbonsaeure MLS000759528MLS001032108MLS001216453MLS001423953SCHEMBL138407CHEMBL1488165DTXCID8026199Pidotimod, >=98% (HPLC)CHEBI:94618HMS2051C04HMS2231M03HMS3715H06Pharmakon1600-01502322BCP05222HY-B0944Tox21_111865MFCD00867583NSC759841s3106(R)-3-((S)-(5-Oxo-2-pyrrolidinyl)carbonyl)-thiazolidin-4-carbonsaeureAKOS015896354AC-3493AM90280CCG-100832DB11364KS-5229NC00082NSC 759841(R)-3-carbonyl]-1,3-thiazolidine-4-carboxylic Acid4-Thiazolidinecarboxylic acid, 3-((5-oxo-2-pyrrolidinyl)carbonyl)-, (R-(R,S))-NCGC00160516-03BP164260CAS-121808-62-6NS00069185P2147SW197462-2D07261AB00639966-08AB00639966_10AB00639966_11A804790J-502193Q3902720(4R)-3-thiazolidine-4-carboxylic acid;Pidotimod(4R)-3-methyl]-4-thiazolidinecarboxylic acid(4R)-3-{CARBONYL}-1,3-THIAZOLANE-4-CARBOXYLIC ACIDMolecular FormulaC9H12N2O4SMolecular Weight244.27InChIInChI=1S/C9H12N2O4S/c12-7-2-1-5(10-7)8(13)11-4-16-3-6(11)9(14)15/h5-6H,1-4H2,(H,10,12)(H,14,15)/t5-,6-/m0/s1InChI KeyUUTKICFRNVKFRG-WDSKDSINSA-NCanonical SMILESC1CC(=O)N1C(=O)N2CSC2C(=O)O
Patent InformationPatent IDTitlePublication DateEP276752Derivative of thiazolidine-4-carboxylic acid, its preparation and pharmaceutical compositions containing it1988EP450352Liposome formulations of immunomodulating drugs for the topical and aerosol administrations1991EP572942Oral pharmaceutical compositions for specific colon delivery1993US5369131Oral, cutaneous and intravaginal pharmaceutical compositions in the form of foam1994
Physical Data
AppearanceWhite crystalline powder,odorless
Melting Point, °C Solvent (Melting Point) 195 - 197193.99194 - 198195 - 198propan-2-ol, H2O
Density, g·cm-3Measurement Temperature, °C1.50919.84
Spectra
Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Temperature (NMR Spectroscopy), °C Chemical shifts, Spectrum1Hdimethylsulfoxide-d6Chemical shifts, Spectrum13CSpectrum1HChemical shifts, Spectrum1Hdimethylsulfoxide-d6Chemical shifts, Spectrum13Cdimethylsulfoxide-d6Spectrum1Hdimethylsulfoxide-d631.9 - 76.9
Description (IR Spectroscopy)Solvent (IR Spectroscopy)Bandspotassium bromideBands, SpectrumSpectrumKBrBandsKBr
Route of Synthesis (ROS)
Route of Synthesis (ROS) of Pidotimod CAS121808-62-6
ConditionsYieldIn ethanol; water at 5℃; for 5h; Solvent; Temperature; Sonication;Experimental ProcedureTake 0.50g of pidotimod and 40ml of absolute ethanol to form a suspension by sonication. After dissolving the other 0.31g of histidine and 4ml of purified water, slowly add the histidine solution to the aforementioned pidotimod mixture at room temperature. Suspended solution, shake well after adding, cool to 5, continue to stir and crystallize for 5h,Filter to obtain crystalline powder, rinse with absolute ethanol 2 to 3 times,5ml each time, dried under vacuum at 40°C for 10 hours to constant weight to obtain pidotimod histidine salt, crystalline powder, yield 95.2%, purity 99.97% (active ingredient pidotimod detection)95.2%
Safety and Hazards
No data available
Other Data
TransportationUnder room temperature away from lightHS CodeStorageUnder room temperature away from lightShelf Life1 yearMarket Price
DruglikenessLipinski rules componentMolecular Weight244.271logP-1.007HBA6HBD2Matching Lipinski Rules4Veber rules componentPolar Surface Area (PSA)112.01Rotatable Bond (RotB)3Matching Veber Rules2
Quantitative Results1 of 61Comment (Pharmacological Data)Bioactivities presentReferenceDerivative of thiazolidine-4-carboxylic acid, its preparation and pharmaceutical compositions containing it2 of 61Comment (Pharmacological Data)Bioactivities presentReferenceToxicological evaluation of pidotimod 3 of 61Comment (Pharmacological Data)Bioactivities presentReference3-L-(5-thioxo-L-prolyl)thiazolidine-4-carboxylic acid and derivatives therefrom, processes for the preparation thereof and pharmaceutical compositions containing them 4 of 61Comment (Pharmacological Data)Bioactivities presentReferencePidotimod: The state of art5 of 61Comment (Pharmacological Data)Bioactivities presentReferenceEffects of pidotimod soluble powder and immune enhancement of Newcastle disease vaccine in chickens6 of 61Comment (Pharmacological Data)Bioactivities presentReferenceAntimicrobial and immunomodulatory properties and applications of marine-derived proteins and peptides7 of 61Comment (Pharmacological Data)Bioactivities presentReferenceEffects of Pidotimod on recurrent respiratory infections in children with down syndrome: A retrospective Italian study8 of 61Comment (Pharmacological Data)Bioactivities presentReferenceLaser therapy in cutaneous and genital warts: A review article9 of 61Comment (Pharmacological Data)Bioactivities presentReferencePidotimod injection with modified stability, and preparation method thereof10 of 61Comment (Pharmacological Data)Bioactivities presentReferenceAmantadine pidotimod carboxylate and preparation method and application thereof
Use PatternPidotimod CAS#: 121808-62-6 is an immunomodulator suitable for patients with compromised immune function. It can be used for the prevention of acute infections, shorten the duration, and reduce the severity of the disease. It can be used as an adjunctive therapy during the acute infection period.
https://www.chemwhat.com/pidotimod-cas-121808-62-6/
Comments
Post a Comment