IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
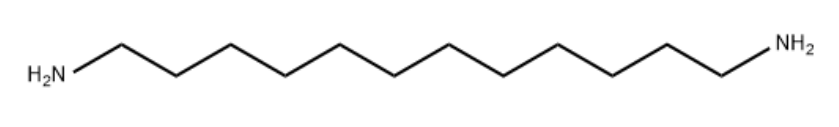
Product Name1,12-Dodecanediamine ineIUPAC Namedodecane-1,12-diamineMolecular StructureCAS Registry Number 2783-17-7EINECS Number220-489-6MDL NumberMFCD00008155Synonyms1,12-Diaminododecane2783-17-71,12-DODECANEDIAMINEDodecane-1,12-diamineDodecamethylenediamineDodecyldiamine1,12-Dodecamethylenediamine1,12-Diamindodecane1,12'-Dodecylenediamine1,12'-Dodecamethylenediamine1,12-DodecylenediamineNSC 55050NSC 59861N12N1,12-n-Dodecanediamine1,12-Diamino-n-dodecaneJ3LM80W9NTCHEMBL69590CHEBI:49385NSC-55050NSC-59861EINECS 220-489-6UNII-J3LM80W9NTBRN 1742765Dodecylenediamine1,12diaminododecanedodecamethylene diamine1,12 diaminododecane1,12-diamino dodecane1,12-diamino-dodecane1,12-dodecane-diamineSCHEMBL274414-04-00-01376 (Beilstein Handbook Reference)1,12-Diaminododecane, 98%DTXSID2044636NSC55050NSC59861ZINC1685531BBL036612BDBM50147574MFCD00008155STL492207AKOS015894529CS-W015599DB-047282D0091FT-0606044F19630A819207Q-200047Q27121625Molecular FormulaC12H28N2Molecular Weight200.36InChIInChI=1S/C12H28N2/c13-11-9-7-5-3-1-2-4-6-8-10-12-14/h1-14H2 InChI KeyQFTYSVGGYOXFRQ-UHFFFAOYSA-N Canonical SMILESC(CCCCCCN)CCCCCN
Physical Data
AppearanceWhite flakes
Melting Point, °C 7068.6968.6867.384266 - 67375
Boiling Point, °CPressure (Boiling Point), Torr115 - 1250.600061524145 - 1482142 - 1445135 - 138318716
Description (Association (MCS))Solvent (Association (MCS))Temperature (Association (MCS)), °CAssociation with compoundAssociation with compoundAssociation with compoundAssociation with compoundH2O22Further physical properties of the complexStability constant of the complex with ...tetrahydrofuran, H2O25
Spectra
Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Temperature (NMR Spectroscopy), °C Frequency (NMR Spectroscopy), MHzChemical shifts1Hdimethylsulfoxide-d6300Chemical shifts1Hdimethylsulfoxide-d6300Chemical shifts, Spectrum1Hchloroform-d1, CD3OD500Chemical shifts, Spectrum13Cchloroform-d1, CD3OD126Chemical shifts, Spectrum1Hwater-d219.94400Spectrum1Hdimethylsulfoxide-d626.34400Spectrum1HCD3OD26.34400
Description (IR Spectroscopy)Solvent (IR Spectroscopy)Bands, Spectrumpotassium bromideBandsneat liquidSpectrumKBr
Description (UV/VIS Spectroscopy)Spectrum
Route of Synthesis (ROS)
Route of Synthesis (ROS) of 1,12-Dodecanediamine CAS 2783-17-7
ConditionsYieldWith hydrogen; sodium methylate In methanol at 130℃; under 37503.8 Torr; for 5h;91.7%Experimental Procedure Thermocouple thermometer,10 g of 1,10-dicyanodecane, 29.4 g of methanol, 0.36 g of a 28 wt% sodium methoxide-methanol solution (in methanol 3.0% by weight), and 0.27 g of a nickel catalyst developed in the same manner as in Example 1 was charged. After charging, hydrogen substitution was carried out, and thereafter the pressure was increased to 5 MPa. The temperature was elevated to an internal temperature of 130 ° C. over about 1 hour after boosting pressure.The reaction started at 130 ° C., and it was confirmed that almost no hydrogen consumption was consumed in about 3 hours. After further reacting for 1 hour, cooling down to room temperature, depressurizing, filtering the reaction solution to remove the catalyst, and washing the filtrate with 100 g of methanol. Methanol concentration was performed from the resultant methanol reaction solution of 1,12-diaminododecane. As a result, the concentration of crude 1,12-diaminododecane obtained after concentration was 10.34 g. The GC area% of 1,12-diaminododecane was 96.8 area%, and the reaction yield of 1,12-diaminododecane calculated from the quantitative value was 91.7%.
Safety and Hazards
No data available
Other Data
TransportationStore in room temperature for long time; Away from light.HS CodeStorageStore in room temperature for long time; Away from light.Shelf LifeHalf of a yearMarket Price
DruglikenessLipinski rules componentMolecular Weight200.368logP3.504HBA2HBD2Matching Lipinski Rules4Veber rules componentPolar Surface Area (PSA)52.04Rotatable Bond (RotB)11Matching Veber Rules1
Quantitative Results1 of 98Comment (Pharmacological Data)Bioactivities presentReferenceβ2- adrenergic receptor agonists2 of 98Comment (Pharmacological Data)Bioactivities presentReferencePropargyl amino compounds3 of 98Comment (Pharmacological Data)Bioactivities presentReferenceBis compounds4 of 98Comment (Pharmacological Data)Bioactivities presentReference5 of 98Comment (Pharmacological Data)Bioactivities presentReferencePYRROLE STUDIES PART 41. REACTIVITY OF 3,4-DIFORMYL-2,5-DIMETHYLPYRROLE WITH DIAMINOALKANES. AN UNUSUAL FORMATION OF 2-AZAFULVENES6 of 98Comment (Pharmacological Data)Bioactivities presentReferenceSynthesis and complexing properties of N,N,N',N'-tetrakis-(8-hydroxy-5-quinolylmethyl)-α,ω-DIAMINOALKANES7 of 98Comment (Pharmacological Data)Bioactivities presentReferenceSELF-ASSEMBLY OF STREPTAVIDIN/BISBIOTIN MONOLAYERS AND MULTILAYERS
Use Pattern1,12-Dodecanediamine CAS#: 2783-17-7 is one of the monomers for the production of nylon 1212.
https://www.chemwhat.com/112-dodecanediamine-cas-2783-17-7/
Comments
Post a Comment