IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
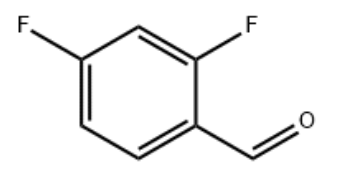
Product Name2,4-Difluorobenzaldehyde(DFBA)IUPAC Name 2,4-difluorobenzaldehyde Molecular StructureCAS Registry Number 1550-35-2EINECS Number216-287-2MDL NumberMFCD00010326Beilstein Registry NumberSynonyms2,4-Difluorobenzaldehyde1550-35-2Benzaldehyde, 2,4-difluoro-2,4-Difluoro-benzaldehydeMFCD00010326CHEMBL4092237EINECS 216-287-22,4difluorobenzaldehyde2,4-difluorbenzaldehyde2,4 difluorobenzaldehyde2.4-difluorobenzaldehyde4,6-Difluorobenzaldehyde2,4-difluoro benzaldehyde2.4-difluoro benzaldehyde2, 4-DifluorobenzaldehydeSCHEMBL969154-Fluoro-2-fluorobenzaldehyde2,4-Difluorobenzaldehyde, 98%DTXSID40165788ACT00455ZINC2004034BDBM50234268STK510038TD1185AKOS000119855AC-3833PS-9013BP-21347DB-023754AM20060046D2839FT-0610082FT-0651771EN300-21300W-108024F2190-0609Z104495222Molecular FormulaC7H4F2OMolecular Weight142.103InChIInChI=1S/C7H4F2O/c8-6-2-1-5(4-10)7(9)3-6/h1-4H InChI KeyWCGPCBACLBHDCI-UHFFFAOYSA-N Canonical SMILESC1=CC(=C(C=C1F)F)C=O
Patent InformationPatent IDTitlePublication DateCN1141059716-(benzo 1, 3 dioxopentacyclic)-4phenyl-6H-1, 3-thiazine-2-amine derivative and application2022CN113024414Method for efficiently synthesizing fluorine-containing compound2021CN110590665Camphoric acid-based acylhydrazone derivative, preparation method and applications thereof2019WO2016/149311FUNGICIDAL PYRAZOLES2016
Physical Data
AppearanceColorless to pale yellow transparent liquid
Melting Point, °C Solvent (Melting Point) 2 - 3
Boiling Point, °CPressure (Boiling Point), Torr80 - 9030 - 35824250 - 521662 - 631165 - 6617
Density, g·cm-3Reference Temperature, °CMeasurement Temperature, °C1.144251.24-1901.24
Spectra
Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Temperature (NMR Spectroscopy), °C Frequency (NMR Spectroscopy), MHzChemical shifts1Hchloroform-d1400Chemical shifts13Cchloroform-d1100Chemical shifts, Spectrum1Hchloroform-d1400Chemical shifts, Spectrum13Cchloroform-d1101
3-Aminopyridine CAS#: 462-08-8 HNMR
Description (IR Spectroscopy)Solvent (IR Spectroscopy)Temperature (IR Spectroscopy), °CBandspotassium bromideBandspotassium bromideFT-IR-Difference Spectroscopy, Bands, SpectrumBands, SpectrumBandsBandsnujol
Description (UV/VIS Spectroscopy)Solvent (UV/VIS Spectroscopy)Comment (UV/VIS Spectroscopy)Spectrumgas220 - 250 nm
Route of Synthesis (ROS)
Route of Synthesis (ROS) of 2,4-Difluorobenzaldehyde(DFBA) CAS1550-35-2
ConditionsYieldWith sodium tetrahydroborate In methanol at 0℃; Inert atmosphere;100%Stage #1: 2,4-difluorobenzaldehyde With C36H44F4N2Ni2P2; diphenylsilane In tetrahydrofuran at 45℃; for 3h; Schlenk technique; Inert atmosphere;Stage #2: With sodium hydroxide In tetrahydrofuran; methanol at 60℃; for 24h; Schlenk technique; Inert atmosphere;92%With methanol; sodium tetrahydroborate at 0℃; for 1h;88%Experimental Procedure Example 13; Preparation of Intermediates (1-(Bromomethyl)-2,4-difluorobenzene) (I-1)To a stirred solution of 2,4-difluorobenzaldehyde (500 mg, 3.52 mmol) in CH3OH (8 mL) was added NaBH4 (266 mg, 7.04 mmol) portion wise at 0° C., and the reaction mixture was stirred at 0° C. for 1 h. After completion of the reaction (by TLC), CH3OH was removed under reduced pressure, diluted with ice-cold H2O (40 mL) and extracted with EtOAc (2×20 mL). The combined organic layers were washed with H2O (40 mL) and brine (40 mL), dried over anhydrous Na2SO4 and concentrated under reduced pressure to obtain the crude material. Purification by silica gel column chromatography eluting with 10% EtOAc/hexanes afforded the alcohol G (450 mg, 3.12 mmol, 88%) as colorless liquid. 1H NMR (200 MHz, CDCl3): δ 7.45-7.33 (m, 1H), 6.83-6.75 (m, 2H), 4.72 (s, 2H), 1.79 (br s, OH).
Safety and Hazards
Pictogram(s)SignalDangerGHS Hazard StatementsH300: Fatal if swallowed H301: Toxic if swallowed H311: Toxic in contact with skin H315: Causes skin irritation H319: Causes serious eye irritation H331: Toxic if inhaled H335: May cause respiratory irritation H373: Causes damage to organs through prolonged or repeated exposure H400: Very toxic to aquatic life H410: Very toxic to aquatic life with long lasting effects Information may vary between notifications depending on impurities, additives, and other factors. Precautionary Statement CodesP260, P261, P264, P270, P271, P273, P280, P301+P310, P302+P352, P304+P340, P305+P351+P338, P311, P312, P314, P321, P322, P330, P332+P313, P337+P313, P361, P362, P363, P391, P403+P233, P405, and P501(The corresponding statement to each P-code can be found at the GHS Classification page.)
Other Data
TransportationKeep away from heat, sparks, and flame. Keep away from sources of ignition. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances. Do not expose to air. Store under an inert atmosphereHS CodeStorageKeep away from heat, sparks, and flame. Keep away from sources of ignition. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances. Do not expose to air. Store under an inert atmosphereShelf Life1 yearMarket PriceUSD 300/kg
DruglikenessLipinski rules componentMolecular Weight142.105logP2.04HBA1HBD0Matching Lipinski Rules4Veber rules componentPolar Surface Area (PSA)17.07Rotatable Bond (RotB)1Matching Veber Rules2
Quantitative Results1 of 59Comment (Pharmacological Data)Bioactivities presentReference4-HYDROXYPIPERIDINE DERIVATIVES HAVING ANTIARRHYTHMIC EFFECT2 of 59Comment (Pharmacological Data)Bioactivities presentReferenceTriazolo-pyridazine derivatives as ligands for GABA receptors3 of 59Comment (Pharmacological Data)Bioactivities presentReference4 of 59Comment (Pharmacological Data)Bioactivities presentReferenceSynthesis and calcium antagonistic activity of diethyl styrylbenzylphosphonates5 of 59Comment (Pharmacological Data)Bioactivities presentReferenceSynthesis and antitumor activities of novel 5-deazaflavin-sialic acid conjugate molecules6 of 59Comment (Pharmacological Data)Bioactivities presentReference3-Trifluoromethyl-4-nitro-5-arylpyrazoles are novel KATP channel agonists7 of 10Comment (Pharmacological Data)Bioactivities presentReferenceCross reactions of cyanoacetic acid derivatives with carbonyl compounds 3. One-step synthesis of substituted 2-amino-5-oxo-4,5-dihydropyrano chromenes
Use Pattern2,4-Difluorobenzaldehyde(DFBA) CAS#: 1550-35-2 isused in medicine, pesticide, liquid crystal material intermediates.
https://www.chemwhat.com/24-difluorobenzaldehydedfba-cas-1550-35-2/
Comments
Post a Comment