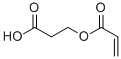
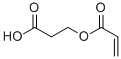
IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
Product Name2-CARBOXYETHYL ACRYLATEIUPAC Name3-prop-2-enoyloxypropanoic acidMolecular StructureCAS Registry Number 24615-84-7MDL NumberMFCD00040709Synonyms2-Carboxyethyl acrylate24615-84-72-Propenoic acid, 2-carboxyethyl esterHydracrylic acid, acrylatecarboxyethyl acrylate3-(Acryloyloxy)propionic acidKM1E1216WRDTXSID2027834ACRYLIC ACID DIMEREBECRYL .BETA.-CEAARONIX M 566C 800 (ACRYLATE)DTXCID807834ACRYLIC ACID 2-CARBOXYETHYL ESTER.BETA.-HYDROXYPROPIONIC ACID ACRYLIC ESTEREBECRYL BETA-CEACARBOXYETHYL ACRYLATE BETA-HYDROXYPROPIONIC ACID ACRYLIC ESTER246-359-93-prop-2-enoyloxypropanoic acid3-(acryloyloxy)propanoic acidbeta-Carboxyethyl acrylate2-Carboxyethyl 2-propenoate2-carboxyethylacrylateHydracrylic acid acrylateSipomer B-CEA3-Acryloyloxypropionic acidCAS-24615-84-7beta-(Acryloyloxy)propionic acidEINECS 246-359-9UNII-KM1E1216WRbeta-carboxyethylacrylatebeta carboxyethyl acrylatebeta-acryloxypropionic acidSCHEMBL27385CHEMBL31851073-(prop-2-enoyloxy)propanoic acidTox21_202236Tox21_303489AKOS006377408Beta-carboxyethyl acrylate,distributionNCGC00249195-01NCGC00257334-01NCGC00259785-01AS-77136.BETA.-(ACRYLOYLOXY)PROPIONIC ACIDCS-0346107NS00019712EN300-202121F71247Q272823302-Carboxyethyl acrylate, contains 900-1100 ppm MEHQ as inhibitor7BCMolecular FormulaC6H8O4Molecular Weight144.12InChIInChI=1S/C6H8O4/c1-2-6(9)10-4-3-5(7)8/h2H,1,3-4H2,(H,7,8)InChI KeyCYUZOYPRAQASLN-UHFFFAOYSA-NSMILESC=CC(=O)OCCC(=O)O
Patent InformationPatent IDTitlePublication DateUS2011/65827NOVEL HOLOGRAPHIC MEDIA AND PHOTOPOLYMERS2011US2007/225522Method for Producing Carboxyl Group-Containing Water-Soluble Polymer2007WO2005/80308CLEAVING OLIGOMERIC (METH)ACRYLIC ACID IN THE LIQUID PHASE AND UNDER PRESSURE2005
Physical Data
AppearanceTransparent liquid
Boiling Point, °CPressure (Boiling Point), Torr146 - 14816136101327
Density, g·cm-3Reference Temperature, °CMeasurement Temperature, °C1.24201.20192020
Spectra
Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Frequency (NMR Spectroscopy), MHzChemical shifts, Spectrum1Hdimethylsulfoxide-d6Spectrum1Hwater-d2400Chemical shifts1HCDCl3
Description (IR Spectroscopy)Bands
Route of Synthesis (ROS)
Route of Synthesis (ROS) of Lentinan CAS 37339-90-5
ConditionsYieldWith oxalyl dichloride; 10H-phenothiazine; 9,10-phenanthrenequinone; N,N-dimethyl-formamide In dichloromethane at 0 - 20℃; under 200 Torr;Experimental ProcedureCEA from Example 7 (51 g; ~ 0.35 mole) and dimethyl formamide (DMF; 0.2 mL; 0.26 mmole) were dissolved in CH2Cl3 (100 mL). The CEA solution was added slowly (over 2 hours) to a stirred solution of oxalyl chloride (53 mL; 0.61 mole), DMF (0.2 mL; 2.6 mmole), anthraquinone (0.5 g; 2.4 mmole), phenothiazine (0.1 g, 0.5 mmole), and CH2Cl3 (75 mL) in a 500 mL round bottom flask in an ice bath at 200 mm pressure. A dry ice condenser was used to retain the CH2Cl3 in the reaction flask. After the addition was complete the reaction was stirred at room temperature overnight. The weight of reaction solution was 369 g. A sample of the CEA-Cl (Compound 7) reaction solution (124 mg) was treated with 1,4- dibromobenzene (DBB, 6.85 mg) evaporated and dissolved in CDCl3: 1H NMR (CDCl3, 400 MHz) δ 7.38 (s, 4H; DBB internal std.), 6.45 (d, IH, J = 17.4 Hz), 6.13 (dd, IH, J = 17.4, 10.4 Hz), 5.90 (d, IH, J = 10.4 Hz), 4.47 (t, 2H, J = 5.9 Hz), 3.28 (t, 2H, J = 5.9). The spectra was consistent with the desired product. There was 0.394 mole DBB for 1.0 mole CEA-Cl by integration, which gave a calculated yield of 61%. Commercially available CEA (426 g; Aldrich) was reacted with oxalyl chloride (532 mL) in a procedure similar to the one listed above. The residue CEA- Cl (490 g) was distilled using an oil bath at 1400C at a pressure of 18 mm Hg. The distillate temperature reached 980C and 150 g of distillate was collected. The distillate was redistilled at 18 mm Hg at a maximum bath temperature of 1200C. The temperature range for the distillate was 300C to 700C which gave 1 1 g of material. The distillate appeared to be 3-chloro-3-oxopropyl 3-chloropropanoate. The residue of the second distillation (125 g; 26 % of theory) was used in Example 9.A 61%B 26%
Safety and Hazards
Pictogram(s)SignalDangerGHS Hazard StatementsH302 (71.2%): Harmful if swallowed H312 (57.7%): Harmful in contact with skin H314 (77.8%): Causes severe skin burns and eye damage H315 (14.8%): Causes skin irritation H318 (73%): Causes serious eye damage H319 (14.8%): Causes serious eye irritation Precautionary Statement CodesP260, P264, P264+P265, P270, P280, P301+P317, P301+P330+P331, P302+P352, P302+P361+P354, P304+P340, P305+P351+P338, P305+P354+P338, P316, P317, P321, P330, P332+P317, P337+P317, P362+P364, P363, P405, and P501(The corresponding statement to each P-code can be found at the GHS Classification page.)
Other Data
TransportationUnder the room temperature and away from lightHS CodeStorageUnder the room temperature and away from lightShelf Life2 yearsMarket Price
DruglikenessLipinski rules componentMolecular Weight144.127logP0.073HBA4HBD1Matching Lipinski Rules4Veber rules componentPolar Surface Area (PSA)63.6Rotatable Bond (RotB)5Matching Veber Rules2
Use PatternUsed as an excellent active diluent in UV curing coatings, adhesives, and ink systems to improve adhesion. Used as a special monomer for emulsion polymerization, adhesives, and coating resin production. https://www.chemwhat.com/2-carboxyethyl-acrylate-cas-24615-84-7/
Comments
Post a Comment