
IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
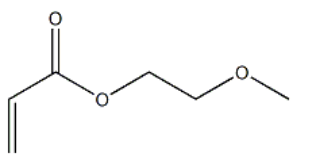
Product NamePOLY(ETHYLENE GLYCOL) METHYL ETHER ACRYLATEIUPAC Name2-methoxyethyl prop-2-enoate Molecular StructureCAS Registry Number 32171-39-4MDL NumberMFCD00803685Synonyms2-METHOXYETHYL ACRYLATE3121-61-7Methoxyethyl acrylate2-Propenoic acid, 2-methoxyethyl esterEthylene glycol methyl ether acrylateMethyl cellosolve acrylateEthylene glycol monomethyl ether acrylate2-Methoxyethanol, acrylateSipomer MCAAcrylic acid, 2-methoxyethyl esterGlycol monomethyl ether acrylateAcrylic acid, 2-methoxyethoxy esterEthanol, 2-methoxy-, acrylate2-Propenioc acid, 2-methoxyethyl esterNSC 24153Ageflex MEAEINECS 221-499-3BRN 1754333AI3-15726NSC-24153515K4I683YDTXSID7025554EC 221-499-3METHOXYETHYL ACRYLATE, 2-2-METHOXYETHYL 2-PROPENOATEDTXCID605554SR 244Acrylic acid, 2-methoxyethyl ester (8CI)221-499-32-methoxyethyl prop-2-enoate32171-39-42-Methoxyethoxy acrylateMFCD008036852-MethoxyethylacrylatemPEG-AcrylateAcryl-PEGMethoxyethyl acrylate(13cp(25 degrees c))MFCD00048149UNII-515K4I683YAcrylic Acid 2-Methoxyethyl EstermethoxyethylacrylatemPEG-AC2-methyoxyethyl acrylateSCHEMBL38483WLN: QVYU1&2O1WLN: 1U1VO2O1HFCUBKYHMMPGBY-UHFFFAOYSA-NSC24153AKOS005206914CS-W011111BS-42697SY074897Ethylene glycol methyl ether acrylate, 98%NS000042742-Methoxyethyl Acrylate (stabilized with MEHQ)E77141F778542-Methoxyethyl Acrylate, (stabilized with MEHQ)EN300-1718841Q27260866Ethylene Glycol Monomethyl Ether Acrylate (stabilized with MEHQ)InChI=1/C6H10O3/c1-3-6(7)9-5-4-8-2/h3H,1,4-5H2,2H3Ethylene glycol methyl ether acrylate, contains 50-100 ppm MEHQ as inhibitor, 98%ne, 3-amino-;β-Aminopyridine462-08-8Molecular FormulaC6H10O3 Molecular Weight130.139 InChIInChI=1S/C6H10O3/c1-3-6(7)9-5-4-8-2/h3H,1,4-5H2,2H3InChI KeyHFCUBKYHMMPGBY-UHFFFAOYSA-NSMILESCOCCOC(=O)C=C
Patent InformationPatent IDTitlePublication DateCN117924192Conjugated olefin derivative as well as preparation method and application thereof2024CN1140576392-aryl pentadienoic acid ester containing terminal olefin and synthesis method thereof2022CN1135829463-aryl-5-thio-1,3,4-thiadiazole-2-thione derivative as well as preparation method and application thereof2021CN110668942Alcohol-substituted (E, E)-configuration dendritic conjugated diene derivative and preparation method thereof2020CN110642737Amide-substituted (E,E)-configuration dendritic conjugated diene derivative and preparation method thereof2020CN110818630Synthesis method of conjugated (E)-3-cycloalkenyl acrylate derivative2020CN110642752Alkenylation product of allylic alcohol carbamate compound and synthesis method thereof2020
Physical Data
AppearanceColorless transparent liquid
Boiling Point, °CPressure (Boiling Point), Torr5612
Density, g·cm-3Reference Temperature, °CMeasurement Temperature, °C1.0131420
Spectra
Description (IR Spectroscopy)Spectrum
Route of Synthesis (ROS)
Route of POLY(ETHYLENE GLYCOL) METHYL ETHER ACRYLATE CAS 32171-39-4
ConditionsYieldWith tetrabutoxytitanium; 4-methoxy-phenol In o-xylene at 80 - 90℃; under 75.0075 Torr;Experimental ProcedureIn a 500 ml four-necked flask equipped with a distillation apparatus, a thermometer, and a stirrer,1,4-butanediol monoglycidyl ether (hereinafter referred to as "14 BDMGE"100.0 g (purity: 93.7%, 0.64 mol in terms of purity)),125.1 g (0.96 mol) of 2-methoxyethyl acrylate,200.0 g of o-xylene and 0.03 g of MEHQ were added to prepare a raw material mixture solution(Total 425.1 g) were prepared.When the moisture content of this raw material mixture liquid was measured with a Karl Fischer moisture meter, it was 503 ppm, and the total amount of moisture contained was 0.21 g (0.012 mol). To the above raw material mixture solution, 4.4 g (0.013 mol) of TBT was added,While heating the solution under reduced pressure while reducing the pressure to 100 hPa, transesterification reaction was carried out at a reaction solution temperature of 80 to 90 ° C. while o-xylene and produced 2-methoxyethanol were distilled out of the reaction system.The temperature of the distillation gas was 57 to 74 ° C.Finally, 169.9 g of the fraction was withdrawn in 6 hours and the reaction was terminated to obtain 255.2 g of a reaction solution containing 4 HBAGE (46.3 wt%).The purity-converted quantitative yield of 4 HBAGE is 92.0%, and the molar ratio of the total amount of moisture in this reaction system is 0.9 times the TBT used. To the above reaction solution, 140.0 g of water was added, heated to 60 ° C. under normal pressure with stirring, and heated and hydrolyzed at 60 ° C. for 1 hour as it was. The reaction solution was cooled and suction filtered using a filter equipped with Celite to remove insoluble catalyst. The filtrate was separated into an organic layer and an aqueous layer. The organic layer containing 4 HBAGE was concentrated under reduced pressure using a rotary evaporator to obtain 130.8 g of 4 HBAGE crude product (purity 94.0%). The purity-converted quantitative yield of 4 HBAGE was 95.8%, and the content of titanium derived from TBT used as a catalyst was 2 ppm or less.50.0 g of the above 4 HBAGE crude product (130.8 g) was fractionated, 0.08 g of CBC was added thereto, and purification was carried out by simple distillation under reduced pressure. As a result, high purity 4 HBAGE (purity 96.9%) was obtained.The purity-converted quantitative yield of 4 HBAGE is 83.7% in terms of the consistent yield from the transesterification reaction. Conditions at the time of fractional distillation were 0.4 to 0.6 hPa in the degree of vacuum, 103 to 114 ° C. in the bottom liquid temperature, 93 to 95 ° C. in the distillation gas temperature, about 1 hour in distillation time there were. No polymer was observed in the distillation residue and in the distillation system.83.7%
Safety and Hazards
Pictogram(s)SignalDangerGHS Hazard StatementsH226 (89.4%): Flammable liquid and vapor H302 (89.4%): Harmful if swallowed H311+H331 (73.1%): Toxic in contact with skin or if inhaled. H311 (89.4%): Toxic in contact with skin H314 (74%): Causes severe skin burns and eye damage H315 (25%): Causes skin irritation H317 (77.9%): May cause an allergic skin reaction H318 (37.5%): Causes serious eye damage H319 (25%): Causes serious eye irritation H331 (78.8%): Toxic if inhaled H332 (10.6%): Harmful if inhaled H335 (11.5%): May cause respiratory irritation H341 (19.2%): Suspected of causing genetic defects H360 (57.7%): May damage fertility or the unborn child H360FD (17.3%): May damage fertility; May damage the unborn child H373 (63.5%): May causes damage to organs through prolonged or repeated exposure H411 (15.4%): Toxic to aquatic life with long lasting effects H412 (72.1%): Harmful to aquatic life with long lasting effects Precautionary Statement CodesP203, P210, P233, P240, P241, P242, P243, P260, P261, P262, P264, P264+P265, P270, P271, P272, P273, P280, P301+P317, P301+P330+P331, P302+P352, P302+P361+P354, P303+P361+P353, P304+P340, P305+P351+P338, P305+P354+P338, P316, P317, P318, P319, P321, P330, P332+P317, P333+P317, P337+P317, P361+P364, P362+P364, P363, P370+P378, P391, P403+P233, P403+P235, P405, and P501(The corresponding statement to each P-code can be found at the GHS Classification page.)
Other Data
TransportationStore at room temperature for long time, sealed and keep in a dry place.HS CodeStorageStore at room temperature for long time, sealed and keep in a dry place.Shelf Life1 yearMarket Price
DruglikenessLipinski rules componentMolecular Weight130.144logP-0.343HBA3HBD0Matching Lipinski Rules4Veber rules componentPolar Surface Area (PSA)35.53Rotatable Bond (RotB)5Matching Veber Rules2
Use PatternMPEG-AC CAS 32171-39-4 finds extensive applications in UV-curable systems and related fields. It is widely used as a key raw material in high-performance UV-curable adhesives, particularly for laminated glass, flexible displays, and optical device bonding. In UV-curable systems, it acts as a modifier to adjust curing speed, flexibility, and adhesion performance, optimizing the physical and chemical properties of the final product. It serves as a monomer in polymerization reactions for the synthesis of specialty acrylic resins or cyclohexyl-based compounds. https://www.chemwhat.com/polyethylene-glycol-methyl-ether-acrylate-cas-32171-39-4/
Comments
Post a Comment