
IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
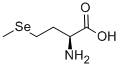
Product NameL-(+)-SelenomethionineIUPAC Name(2S)-2-amino-4-methylselanylbutanoic acidMolecular StructureCAS Registry Number 3211-76-5MDL NumberMFCD00037210SynonymsL-selenomethionine3211-76-5Seleno-L-methionineSelenium-L-methionineL-SelenomethioninumSeMet(S)-2-Amino-4-(methylselanyl)butanoic acid(2S)-2-amino-4-methylselanylbutanoic acid(S)-2-Amino-4-(methylseleno)butyric acid(S)-2-Amino-4-(methylseleno)butanoic acid(2S)-2-amino-4-(methylseleno)butanoic acidL-(+)-SelenomethionineMFCD00037210Butyric acid, 2-amino-4-(methylselenyl)-, L-Butanoic acid, 2-amino-4-(methylseleno)-, (S)-NSC-760370964MRK2PELDTXSID8046824CHEBI:30021NCGC00181044-01Selenomethionine MSE(2s)-2-amino-4-(methylselanyl)butanoic acidSelenoSource AF 2000C5H11NO2SeUNII-964MRK2PELButanoic acid, 2-amino-4-(methylseleno)-, (2S)-(2S)-2-amino-4-methylselanyl-butanoic acidMegavite RXSeleno-DL-methionine;DL-Selenomethionine(2S)-2-Amino-4-(methylseleno)-butanoic acid; L-2-Amino-4-(methylselenyl)-butyric acid; Seleno-L-methionineMethionine, seleno-L-Seleno-L-methionineSeleno-D,L-methionineL(+)-Selenomethioninebmse000169SELENOMETHIONINE, L-SCHEMBL63322SELENOMETHIONINE L-SelenoMethionine (Standard)SELENOMETHIONINE CHEMBL113178HY-B1000ARDTXCID6026824L-SELENOMETHIONINE SELENOMETHIONINE HY-B1000ASELENOMETHIONINE SELENOMETHIONINE 2-amino-4-(methylseleno)butanoateHMS3264C04Pharmakon1600-015061632-Amino-4-(methylselenyl)butyrateTox21_112692NSC760370s3973AKOS015853989AKOS015889703AC-5676CCG-207973CS-5178DB11142FS09881NSC 760370(S)-2-Amino-4-(methylseleno)butanoateNCGC00181044-04(2S)-2-amino-4-(methylseleno)butanoate(S)-2-amino-4-(methylseleno)-ButanoateCAS-3211-76-5L-2-amino-4-(methylselenyl)-Butyric acidNS00074298S0442(S)-2-amino-4-(methylseleno)-Butanoic acidSeleno-L-methionine, >=98% (TLC), powderC05335F20529AB01563198_01BRD-K74664543-213-01-2Q27096144.ALPHA.-AMINO-.GAMMA.-(METHYLSELENO)BUTYRIC ACID2905D820-1EFF-4468-93D0-BD1605569F30Selenomethionine, United States Pharmacopeia (USP) Reference StandardMolecular FormulaC5H11NO2Se Molecular Weight196.12InChIInChI=1S/C5H11NO2Se/c1-9-3-2-4(6)5(7)8/h4H,2-3,6H2,1H3,(H,7,8)/t4-/m0/s1InChI KeyRJFAYQIBOAGBLC-BYPYZUCNSA-N SMILESCCC(C(=O)O)N
Patent InformationPatent IDTitlePublication DateCN115475228Application of L-selenomethionine-containing oligopeptide in preparation of antitumor drugs2022CN1096271982-acetonyl seleno-benzamide compound and preparation method and application thereof2019US2015/152139Peptide Tyrosinase Activators2015
Physical Data
AppearanceOff-white to white powderSolubility50mg in 1ml water clear soluble
Melting Point, °C Solvent (Melting Point) 275228275266 - 268aq. HCl
Description (Association (MCS))Solvent (Association (MCS))Temperature (Association (MCS)), °CPartner (Association (MCS))Association with compoundaq. buffer37iron(III) protoporphyrin IX chlorideAssociation with compoundaq. phosphate buffer37human hemoglobin A
Spectra
Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Frequency (NMR Spectroscopy), MHzChemical shifts1Hwater-d2Chemical shifts13Cwater-d2Chemical shifts1Hwater-d2400Chemical shifts1Hwater-d2Chemical shifts13Cwater-d2Chemical shifts, Spectrum1Hwater-d2Chemical shifts, Spectrum1Hheavy water
Description (IR Spectroscopy)Solvent (IR Spectroscopy)Bandspotassium bromideFT-IR, in KBr
Description (UV/VIS Spectroscopy)Spectrum
Route of Synthesis (ROS)
Route of Synthesis (ROS) of L-(+)-Selenomethionine 3211-76-5
ConditionsYieldStage #1: Seleno-L-methionine With sodium hydrogencarbonate In 1,4-dioxane; water for 0.333333h; Cooling with ice;Stage #2: di-tert-butyl dicarbonate In 1,4-dioxane; water at 20℃; for 13h; Cooling with ice;Experimental Procedure2 Example 2: Preparation of Boc-L-selenomethionine0.4 g L-selenomethionine was dissolved in 40 ml dioxane-water (volume ratio 1:1) solution, add 0.5 g NaHCO3. The resulting solution under ice bath was stirred for 20 min. Slowly add dropwise 1.5 eq Boc anhydride in dioxane solution. After ice bath stir for 1 h, raise the temperature to room temperature and react for 12 h. After the reaction is finished, extracted with ethyl acetate twice, the retention of the aqueous phase. For 1 mol/L hydrochloric acid aqueous solution of the aqueous phase is adjusted to pH 1 the left and right, extracted with ethyl acetate twice, rollup organic phase and drying with anhydrous sodium sulfate, concentrated under reduced pressure to obtain the Boc-L-selenomethionine (0.6 g, yield 98%), without further purification, can be directly used for the next step reaction.98%With sodium hydroxide In 1,4-dioxane; water at 20℃; for 20h;Experimental ProcedureTo a solution of LSM in 2M NaOH was added a solution of di-t-butyl pyrocarbonate in dioxane and the mixture was stirred at room temperature for about 20 hrs. Dioxane was removed from the reaction mixture. The aqueous solution was acidified with 10% KHSO4 solution and the liberated Boc-LSM was extracted into ethyl acetate. The ethyl acetate solution was washed with water, dried and concentrated. Precipitation was done using petroleum ether (60-800).With hydrogen In ethyl acetate under 760.051 With sodium hydrogencarbonate In 1,4-dioxane; water at 25℃;Experimental Procedure3.3. Procedure of the Preparation of Selenomethionine-Substituted Curcuminoids and Methionine-Substituted CurcuminoidsGeneral procedure: According to the procedure previously reported , selenomethionine (8) was prepared. Methionine (2) was used as the starting material in a three-pot, seven-step procedure to obtain selenomethionine in 47% yield. In a 100-mL round-bottomed flask, selenomethionine or methionine was dissolved in water and NaHCO3 (3 eq.) was added. A solution of (BOC)2O (1.5 eq.) in dioxane was added to this mixture. The reaction mixture was stirred at 25 °C overnight. The reaction mixture was washed with ethyl acetate. The resulting aqueous layer was acidified to pH 2 with concentrated hydrochloric acid and extracted with ethyl acetate. The combined organic extracts were dried over Na2SO4, filtered and concentrated in vacuo to give 9a or 9b as colorless gums.With sodium hydrogencarbonate In 1,4-dioxane; water at 20℃; for 12h;Experimental Procedure3.3. General Method for Synthesizing Compounds 15-16General procedure: The appropriate amino acid (13 or 14, 1 equiv.) and sodium bicarbonate (3 equiv.) was dissolved in a 1:1 mixture of water and 1,4-dioxane. Di-tert-butyl dicarbonate (1.2 equiv.) was added and the mixture was stirred at room temperature for 12 h. The 1,4-dioxane was removed under reduced pressure and the mixture was extracted with ethyl acetate. Then the solution was acidified using 1 M hydrochloric acid solution and extracted three times with ethyl acetate. The combined organic layers were washed with brine, dried over sodium sulfate, filtered, and the solvent was evaporated.The crude protected amino acids 15-16 were used without any further purification.
Safety and Hazards
Pictogram(s)SignalDangerGHS Hazard StatementsH301 (100%): Toxic if swallowed H331 (100%): Toxic if inhaled H373 (97.9%): May causes damage to organs through prolonged or repeated exposure H400 (99%): Very toxic to aquatic life H410 (99%): Very toxic to aquatic life with long lasting effects Precautionary Statement CodesP260, P261, P264, P270, P271, P273, P301+P316, P304+P340, P316, P319, P321, P330, P391, P403+P233, P405, and P501(The corresponding statement to each P-code can be found at the GHS Classification page.)
Other Data
TransportationUnder the room temperature HS CodeStorageTransport at room temperature, long-term preservation at 0 ~ 5°Shelf Life1 yearMarket Price
DruglikenessLipinski rules componentMolecular Weight94.116logP-0.047HBA2HBD1Matching Lipinski Rules4Veber rules componentPolar Surface Area (PSA)38.91Rotatable Bond (RotB)0Matching Veber Rules2
Use PatternSelenomethionine exhibits antioxidant properties by enhancing glutathione peroxidase activity, thereby protecting cells from oxidative damage such as DNA strand breaks and protein dysfunction. It induces dose-dependent growth inhibition and apoptosis in various human cancer cell lines at micromolar concentrations, while normal fibroblasts are only affected at much higher levels (1 mM). https://www.chemwhat.com/l-selenomethionine-cas-3211-76-5/
Comments
Post a Comment