
IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
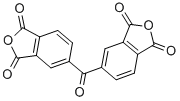
Product Name3,3',4,4'-Benzophenonetetracarboxylic dianhydrideIUPAC Name5-(1,3-dioxo-2-benzofuran-5-carbonyl)-2-benzofuran-1,3-dioneMolecular StructureCAS Registry Number 2421-28-5EINECS Number219-348-1MDL NumberMFCD00005923Synonyms2421-28-53,3',4,4'-Benzophenonetetracarboxylic dianhydride1,3-Isobenzofurandione, 5,5'-carbonylbis-Benzophenonetetracarboxylic dianhydride4,4'-Carbonyldiphthalic anhydrideBenzophenonetetracarboxylic acid dianhydride4,4'-Carbonylbis(phthalic anhydride)5-(1,3-dioxo-2-benzofuran-5-carbonyl)-2-benzofuran-1,3-dioneBenzophenone-3,3',4,4'-tetracarboxylic dianhydride4,4'-Diphthalic anhydride ketonePhthalic anhydride, 4,4'-carbonyldi-Benzophenonetetracarboxylic anhydrideBenzophenonetetracarboxylic acid anhydride5,5'-CARBONYLBIS(ISOBENZOFURAN-1,3-DIONE)3,3',4,4'-Benzophenonetetracarboxylic acid dianhydrideNSC 784803,3',4,4'-Tetracarboxybenzophenone dianhydride5,5'-carbonylbis(2-benzofuran-1,3-dione)3,3',4,4'-BENZOPHENONE TETRACARBOXYLIC DIANHYDRIDEDTXSID3029233Y61GVA8097NSC-784803,3',4,4'-Benzophenonetetracraboxylic Dianhydride3,3',4,4'-Benzophenonetetracarboxylic dianhydride(BTDA)3,3,4,4-Benzophenonetetracarboxylic DianhydrideUNII-Y61GVA8097EINECS 219-348-1MFCD000059231, 5,5'-carbonylbis-EC 219-348-1SCHEMBL169521Benzophenone-3,3':4,4'-tetracarboxylic dianhydrideDTXCID409233CHEMBL31835375-(1,3-dioxoisobenzofuran-5-carbonyl)isobenzofuran-1,3-dionePhthalic anhydride,4'-carbonyldi-NSC78480Tox21_200475BBL000526STK256923AKOS003239423benzophenone tetracarboxylic dianhydrideCS-W009885HY-W0091695,5'-carbonyldiisobenzofuran-1,3-dioneNCGC00248646-01NCGC00258029-01AC-14698CAS-2421-28-5benzophenone tetracarboxylic acid dianhydride3,4,4'-Tetracarboxybenzophenone dianhydrideB0948B4260NS000048833,4,4'-Benzophenonetetracarboxylic dianhydrideD711985,5'-CARBONYLBIS(1,3-ISOBENZOFURANDIONE)Benzophenone-3,3',4,4'-tetracarboxylicdianhydride3,4,4'-Benzophenonetetracarboxylic acid dianhydrideQ-200692Q272942963,3',4,4'-BENZOPHENONETETRACARBOXYLIC ANHYDRIDE3,4,3',4'-BENZOPHENONETETRACARBOXYLIC DIANHYDRIDEBenzophenone-3,3',4,4'-tetracarboxylic dianhydride, 98%3,4,3',4'-BENZOPHENONETETRACARBOXYLIC ACID ANHYDRIDEBENZOPHENONE-3,3',4,4'-TETRACARBOXYLIC ACID ANHYDRIDE3,3',4,4'-Benzophenonetetracarboxylic Dianhydride (purified by sublimation)5,5-Carbonylbis(1,3-isobenzofurandione);4,4-Carbonyldiphthalic anhydride5-(1,3-dioxo-1,3-dihydro-2-benzofuran-5-carbonyl)-1,3-dihydro-2-benzofuran-1,3-dioneBenzophenone-3,3',4,4'-tetracarboxylic dianhydride, purified by sublimation, 96%Benzophenone-3,3 inverted exclamation marka,4,4 inverted exclamation marka-tetracarboxylic dianhydrideMolecular FormulaC17H6O7Molecular Weight322.22InChIInChI=1S/C17H6O7/c18-13(7-1-3-9-11(5-7)16(21)23-14(9)19)8-2-4-10-12(6-8)17(22)24-15(10)20/h1-6HInChI KeyVQVIHDPBMFABCQ-UHFFFAOYSA-NIsomeric SMILESC1=CC2=C(C=C1C(=O)C3=CC4=C(C=C3)C(=O)OC4=O)C(=O)OC2=O
Patent InformationPatent IDTitlePublication DateUS2024/336599PHOTOSENSITIVE POLYAMIC ACID AND DIAZIRINE COMPOSITIONS2024CN1112178392, 6-disubstituted pyrimidine aromatic anhydride as well as synthesis method and application thereof2020CN108503647Benzocyclobutene end-capped imide monomer as well as preparation method and curing method thereof2018JP2017/160131(METH) ACRYL IMIDE COMPOUND AND INK PREPARED THEREWITH2017CN104058993Photosensitive diamine, polyamide acid or the derivatives thereof, the liquid crystal aligning agent, liquid crystal orientation film and liquid crystal display element2017JP2017/202987(METH) ACRYL IMIDE COMPOUND AND INK PREPARED THEREWITH2017JP2017/202988(METH) ACRYL IMIDE COMPOUND AND INK PREPARED THEREWITH2017
Physical Data
AppearanceWhite crystalline powder
Melting Point, °C 228.4223 - 224220308 - 309241221225
Description (Association (MCS))Spectrum of the complex
Spectra
Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Frequency (NMR Spectroscopy), MHzChemical shifts, Spectrum1Hchloroform-d1Spectrum1Hdimethylsulfoxide-d6Chemical shifts, Spectrum13CChemical shifts1Hdimethylsulfoxide-d6300Chemical shifts1Hchloroform-d1600Spectrum1HNMR
Description (IR Spectroscopy)Solvent (IR Spectroscopy)Bands, SpectrumBands, SpectrumATR (attenuated total reflectance), Bands, SpectrumBandspotassium bromideBandsKBrIR
Description (UV/VIS Spectroscopy)Solvent (UV/VIS Spectroscopy)Absorption Maxima (UV/VIS), nmSpectrumacetonitrile300, 580Spectrum297SpectrumUV/VIS
Route of Synthesis (ROS)
Route of Synthesis (ROS) of 3,3',4,4'-Benzophenonetetracarboxylic dianhydride CAS 2421-28-5
ConditionsYieldWith 2,6-bisphenylboronic acid In Butyronitrile for 12h; Reflux;98%With polyphosphoric acid In toluene at 100 - 110℃; for 6.5h; Reagent/catalyst; Temperature;Experimental Procedure1.3; 2.3; 3.3Step (3): add polyphosphoric acid (32g) and toluene (72g) into a 250mL three-necked flask,The intermediate (II) obtained in the above step is stirred under stirring(18.0g, 0.052mol) was added to the reaction solution,The internal temperature was adjusted to 100 to 110°C and stirred for 6.5 hours.Slowly cool down to 20 ~ 30 , filter,The filter cake was rinsed with toluene (30 g) and dried under vacuum at 80°C for 13 hours.14.9 g of yellow final product was obtained, with a purity of 98.9%,Step (3) Molar yield: 92.1%. The three-step total molar yield: 63.0%.92.1%at 220℃; for 1h; Temperature;1.S3; 1.S2; 1; 2.S3; 3.S3; 4.S3; 5.S3; 6.S3; 7.S3; 8.S3S3, the 3,3',4,4'-benzophenone tetracarboxylic acid is placed in a high temperature vacuum sintering furnace and heated to 220°C for melting and dehydration for 1h,get white solid3,3',4,4'-benzophenone tetracarboxylic dianhydride, the calculated yield was 89.6%, and the purity (high performance liquid chromatography) was 99.2%.89.6%
Safety and Hazards
Pictogram(s)SignalWarningGHS Hazard StatementsH319 (100%): Causes serious eye irritation H335 (100%): May cause respiratory irritation Precautionary Statement CodesP261, P264+P265, P271, P280, P304+P340, P305+P351+P338, P319, P337+P317, P403+P233, P405, and P501(The corresponding statement to each P-code can be found at the GHS Classification page.)
Other Data
TransportationUnder room temperature and away from lightHS CodeStorageUnder room temperature and away from light
DruglikenessLipinski rules componentMolecular Weight322.23logP2.578HBA7HBD0Matching Lipinski Rules4Veber rules componentPolar Surface Area (PSA)103.81Rotatable Bond (RotB)2Matching Veber Rules2
Use PatternSynthesis of ketone anhydride, polyimides, used in foams, paints, films, fibers, composites, BTDA containing polyimides have excellent thermal oxidative stability, strength, solvent resistance, flexibility.As an excellent epoxy curing agent, the resin has a high temperature stability, excellent electrical properties, chemical resistance, solvent resistance with BTDA.In the polyester resin, ester formulations, urethane resin, alkyd resin, polybenzimidazole pyrrolidone and other formulations, one BTDA anhydride can be added excellent high temperature and oxidative stability. https://www.chemwhat.com/3344-benzophenonetetracarboxylic-dianhydride-cas-2421-28-5/
Comments
Post a Comment