
IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
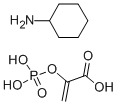
Product NamePhosphoenolpyruvic acid cyclohexylammonium saltIUPAC Namecyclohexanamine;2-phosphonooxyprop-2-enoic acidMolecular StructureCAS Registry Number 10526-80-4EINECS Number234-084-7MDL NumberMFCD00036375Synonyms10526-80-4Cyclohexanamine 2-(phosphonooxy)acrylatePhospho(enol)pyruvic acid cyclohexylammonium saltPHOSPHOENOLPYRUVIC ACID CYCLOHEXYLAMMONIUM SALTEINECS 234-084-7DTXSID30651102-(Phosphonooxy)-2-propenoic acid monopotassium saltMFCD00036375cyclohexylamine; phosphoenolpyruvic acid2-Propenoic acid, 2-phosphonoxy-, monocyclohexylammonium saltPhosphoenolpyruvic acid (cyclohexylammonium salt)C9H18NO6PCyclohexylamine, Compound with 2-Hydroxyacrylic Acid, di-H Phosphate (1:1) (7CI)Cyclohexylammonium Phosphoenolpyruvate; 2-Phosphoenolpyruvate Monocyclohexylammonium(PEP)2-(Phosphonooxy)acrylic acid, compound with cyclohexylamine (1:1)SCHEMBL1611871DTXCID0032944VHFCNZDHPABZJO-UHFFFAOYSA-NAKOS015895036CS-W012420P0758P-6101Phosphoenolpyruvic acid (cyclohexylammoniu salt)Phosphoenolpyruvic acid (cyclohexylammoniu?m salt)Phosphoenolpyruvic acid mono(cyclohexylammonium) saltPhospho(enol)pyruvic acid cyclohexylammonium salt, >=97% (enzymatic)Molecular FormulaC9H18NO6PMolecular Weight267.22InChIInChI=1S/C6H13N.C3H5O6P/c7-6-4-2-1-3-5-6;1-2(3(4)5)9-10(6,7)8/h6H,1-5,7H2;1H2,(H,4,5)(H2,6,7,8)InChI KeyVHFCNZDHPABZJO-UHFFFAOYSA-NIsomeric SMILESC=C(C(=O)O)OP(=O)(O)O.C1CCC(CC1)N
Physical Data
AppearanceWhite Powder
Melting Point, °C 143 - 146144 - 146
Spectra
No data available
Route of Synthesis (ROS)
Route of Synthesis (ROS) of Phosphoenolpyruvic acid cyclohexylammonium salt CAS 10526-80-4
ConditionsYield(i) DCC, Py, (ii) /BRN= 1098229/; Multistep reaction;
Safety and Hazards
Pictogram(s)SignalWarningGHS Hazard StatementsH315 (100%): Causes skin irritation H319 (100%): Causes serious eye irritation H335 (100%): May cause respiratory irritation Precautionary Statement CodesP261, P264, P264+P265, P271, P280, P302+P352, P304+P340, P305+P351+P338, P319, P321, P332+P317, P337+P317, P362+P364, P403+P233, P405, and P501(The corresponding statement to each P-code can be found at the GHS Classification page.)
Other Data
TransportationStore at 2~8 ℃, Sealed and away from light.HS CodeStorageStore at 2~8 ℃, Sealed and away from light.Shelf Life1 year
DruglikenessLipinski rules componentMolecular Weight267.219logPHBA7HBD4Matching Lipinski Rules3Veber rules componentPolar Surface Area (PSA)139.89Rotatable Bond (RotB)3Matching Veber Rules2
Use PatternPhosphoenolpyruvate monocylohexylamine salt (PEP monocylohexylamine salt) is an important organophosphorus compound, primarily used in the fields of biochemistry and pharmaceutical research with the following applications:Substrate in Enzyme Reactions: Phosphoenolpyruvate (PEP) is a key intermediate in the glycolysis pathway. The monocylohexylamine salt form of PEP can be used in vitro to study substrate behavior in enzyme reactions, especially in research involving glycolysis or energy metabolism.Phosphoenolpyruvate Carboxykinase (PEPCK) Studies: PEP is a critical substrate in reactions catalyzed by PEPCK, a key enzyme in the gluconeogenesis pathway. Studying this process is essential for understanding metabolism, diabetes, and other metabolic disorders.ATP Synthesis: In vitro ATP synthesis experiments often use PEP to provide phosphate groups, generating ATP through reactions with ADP. This is highly valuable for research on cellular energy metabolism.Drug Development and Biomedical Research: PEP monocylohexylamine salt may be used in the development of metabolism-related drugs, particularly for research targeting metabolic pathways associated with diseases such as cancer or diabetes. https://www.chemwhat.com/phosphoenolpyruvic-acid-cyclohexylammonium-salt-cas-10526-80-4/
Comments
Post a Comment