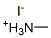
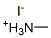
IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
Product Namemethylammonium iodideIUPAC Namemethylazanium;iodide Molecular StructureCAS Registry Number 14965-49-2MDL NumberMFCD28100833SynonymsMethylammonium iodide14965-49-2methylazanium;iodideEINECS 239-037-4methylazanium iodideMethanamine, hydriodideMethyl ammonium iodideMethylamine.hydriodicacidSCHEMBL1534750Methylammonium Iodide, anhydrousDB-221235NS00086090Molecular FormulaCH6INMolecular Weight158.97InChIInChI=1S/CH5N.HI/c1-2;/h2H2,1H3;1HInChI KeyLLWRXQXPJMPHLR-UHFFFAOYSA-N Canonical SMILESC.
Patent InformationPatent IDTitlePublication DateWO2023/180219A METHOD FOR SYNTHESIS OF HALIDE SALTS2023CN113845428Preparation method of perovskite material powder2021WO2018/169373METHOD OF PREPARING LUMINESCENT NANO-SHEET, LUMINESCENT NANO-SHEET MATERIAL, LUMINESCENT NANO-SHEET FILM, BACK LIGHT, AND LIQUID CRYSTAL DISPLAY APPARATUS2018WO2015/32748AMORPHOUS MATERIAL AND THE USE THEREOF2018
Physical Data
AppearanceWhite powder
Melting Point, °C Solvent (Melting Point) 220263 - 265260 - 270aq. ethanol63 - 64ethanol, CHCl3
Density, g·cm-32.22.235
Spectra
Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Frequency (NMR Spectroscopy), MHzChemical shifts1Hdimethylsulfoxide-d6Chemical shifts13Cdimethylsulfoxide-d6NOE (Nuclear Overhauser Effect), Chemical shifts, Spectrum1Hdimethylsulfoxide-d6NOE (Nuclear Overhauser Effect), Chemical shifts, Spectrum13Cdimethylsulfoxide-d6Chemical shifts, Spectrum1Hwater-d2400.1Chemical shifts, Spectrum13Cwater-d2100.6Spectrum13Cacetonitrile
Description (IR Spectroscopy)Solvent (IR Spectroscopy)BandsSpectrumATR (attenuated total reflectance), SpectrumSpectrumpotassium bromideBands, SpectrumBandsSpectrum
Description (UV/VIS Spectroscopy)Solvent (UV/VIS Spectroscopy)Absorption Maxima (UV/VIS), nmSpectrumSpectrumneat (no solvent)Spectrumisopropyl alcohol290, 360
Route of Synthesis (ROS)
Route of Synthesis (ROS) of methylammonium iodide CAS 14965-49-2
ConditionsYieldWith hydrogen iodide In methanol; water at 0℃; for 2h;Experimental ProcedurePreparation of CH3NH3IIn a 500 mL round bottom flask, a methanol solution of methylamine (60.4 mL, 40%, 592mmol; Wako Pure Chemical Industries, Ltd.) was added dropwise over 10 min to an aqueous solution of HI (65.0 mL, 57 wt%, 492 mmol; Wako Pure Chemical Industries Ltd., Japan) at 0 °C, and stirried for 2 h. The resulting white solids were collected by filtration, and dried in vacuo for 24 h at 60 °C. The obtained crude CH3NH3I was purified by recrystallization from a slow diffusion of dry diethyl ether (dehydrated, Kanto Chemical Co. Ltd., Japan, further purified with Glass contour) into a dry methanol (super dehydrated, Wako Pure Chemical Industries Ltd., Japan) solution of CH3NH3I. The resulting CH3NH3I (75.6 g, 476 mmol, 97%yield) was isolated by filtration in an inert glovebox under N2 as colorless crystalline platelets.97%With hydrogen iodide; phosphorous acid In ethanol; water at 0℃;89%With hydrogen iodide In water at 0 - 20℃; for 0.333333h;88%
Safety and Hazards
Pictogram(s)SignalWarningGHS Hazard StatementsH302 (97.6%): Harmful if swallowed H315 (100%): Causes skin irritation H319 (100%): Causes serious eye irritation H335 (97.6%): May cause respiratory irritation Precautionary Statement CodesP261, P264, P264+P265, P270, P271, P280, P301+P317, P302+P352, P304+P340, P305+P351+P338, P319, P321, P330, P332+P317, P337+P317, P362+P364, P403+P233, P405, and P501(The corresponding statement to each P-code can be found at the GHS Classification page.)
Other Data
TransportationUnder room temperature away from light, Oxygen-proof, water-proof, light-proof, low-humidity, dry storage.HS CodeStorageUnder room temperature away from light, Oxygen-proof, water-proof, light-proof, low-humidity, dry storage.Shelf Life1 yearMarket Price
DruglikenessLipinski rules componentMolecular Weight158.97logP0.484HBA1HBD1Matching Lipinski Rules4Veber rules componentPolar Surface Area (PSA)26.02Rotatable Bond (RotB)0Matching Veber Rules2
Use PatternMethylammonium iodide (MAI), based on alkyl halides like iodides and bromides, serves as a crucial precursor for the preparation of perovskite materials used in photovoltaic applications. When combined with lead iodide (PbI₂), MAI plays a key role in forming methylammonium lead iodide perovskites (MAPbI₃), which significantly impact the morphology and crystallization of the perovskite structure. This modification of the material's structure can improve its optoelectronic properties, making it highly efficient for energy-harvesting applications.Perovskite materials are known for their excellent light absorption, charge carrier mobility, and tunable bandgaps, making them ideal for use in next-generation energy devices. In particular, MAI-based perovskites are central to the development of perovskite solar cells (PSCs), which have shown rapid efficiency improvements in recent years. In addition to their potential in solar cells, these perovskite materials are also being explored in the production of light-emitting diodes (LEDs), offering a promising alternative for high-efficiency lighting and display technologies.As the world shifts towards renewable energy, perovskite-based devices, enabled by precursors like methylammonium iodide, are poised to play a critical role in advancing sustainable energy solutions. https://www.chemwhat.com/methylammonium-iodide-cas-14965-49-2/
Comments
Post a Comment