
IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
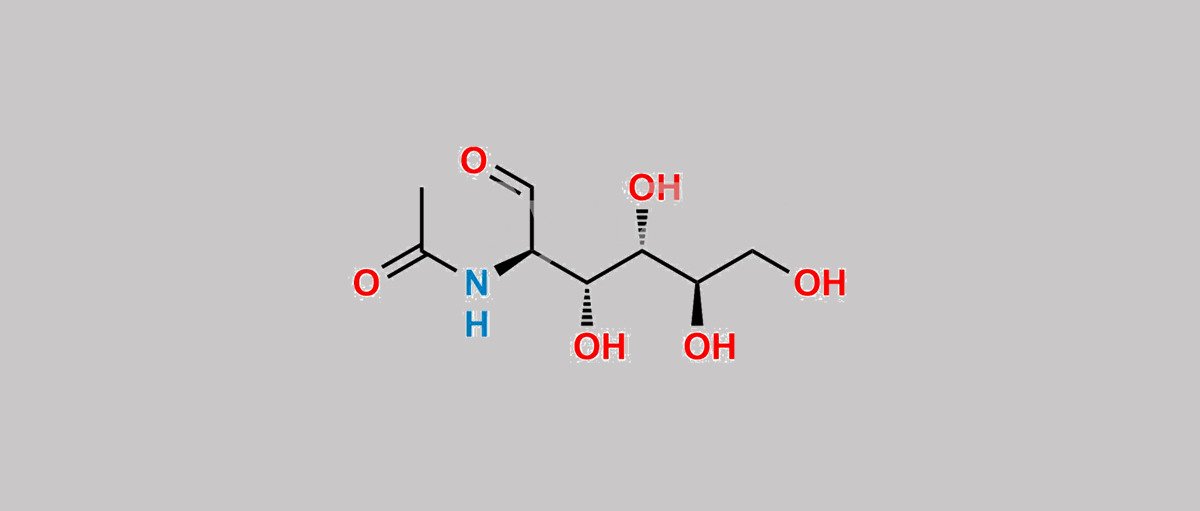
Product NameN-Acetyl GlucosamineMolecular StructureCAS Registry Number7512-17-6EINECS Number231-368-2MDL NumberMFCD00061615Synonyms7512-17-6Marine SweetGreenNAGBio-NAGaldehydo-N-acetyl-D-glucosamineacetyl-glucosamineN acetylglucosamineCCRIS 9357DTXSID3045855N-acetamide2-Acetamido-2-deoxyglucoseEINECS 231-368-2UNII-V956696549NSC 400525NSC 5243442 Acetamido 2 DeoxyglucoseN-((2R,3R,4S,5R)-3,4,5,6-Tetrahydroxy-1-oxohexan-2-yl)acetamideAI3-51898DTXCID5032369D-Glucose, 2-(acetylamino)-2-deoxy-V956696549NSC-400525NSC-524344D-Glucose, 2-acetamido-2-deoxy-N-ACETYLGLUCOSAMINE (USP-RS)N-ACETYLGLUCOSAMINE MFCD00136044NCGC00159338-02NCGC00159338-03N-acetylglucosaminesN Acetyl D GlucosamineGlucosamine, N-acetyl-SCHEMBL19900MLS006011606N-ACETYL GLUCOSAMINE D-2 Acetamido 2 Deoxy D GlucoseCHEMBL4303483N-ACETYLGLUCOSAMINE CHEBI:17411CHEBI:59640N-ACETYLGLUCOSAMINE HY-A0132Tox21_111344BBL033994MFCD00061615N-ACETYLGLUCOSAMINE STK801800AKOS005622647DB00141NCGC00159338-05BP-13072DS-18661SMR002121580CAS-7512-17-6CS-0017447S6257A-1200AB01325684-02EN300-7414561GLUCOPYRANOSE, 2-ACETAMIDO-2-DEOXY-, D-N-Acetyl-D-glucosamine-Agarose, saline suspensionBRD-K80653534-001-01-7Q320302101186B229-E28C-4CF9-8146-EC06A584F821GLUCOSAMINE SULFATE POTASSIUM CHLORIDE IMPURITY A 27555-50-6Molecular FormulaC8H15NO6Molecular Weight221.21InChIInChI=1S/C8H15NO6/c1-4(12)9-5(2-10)7(14)8(15)6(13)3-11/h2,5-8,11,13-15H,3H2,1H3,(H,9,12)/t5-,6+,7+,8+/m0/s1InChI KeyMBLBDJOUHNCFQT-LXGUWJNJSA-NIsomeric SMILESCC(=O)N(C=O)(((CO)O)O)O
Physical Data
AppearanceWhite powder
Melting Point, °C Solvent (Melting Point) 218186 - 190ethanol, diethyl ether197205 - 207H2O205
Description (Association (MCS))Temperature (Association (MCS)), °CPartner (Association (MCS))Association with compound25H2O
Spectra
Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Chemical shifts13CD2OSpin-lattice relaxation time (T1)Spin-spin relaxation time (T2)NMR
Route of Synthesis (ROS)
Route of Synthesis (ROS) of N-Acetyl-D-glucosamine CAS 7512-17-6
ConditionsYieldWith Biolacta N5 In N,N-dimethylhexanamide at 30℃; pH=5; aq. buffer; Enzymatic reaction; regioselective reaction;Experimental Procedure4.5. General procedure for transglycosylation reactions using glycoconjugatesGeneral procedure: A solution of 51.2 mg (0.17 M) p-nitrophenyl-β-d-galactopyranoside (donor) and 188 mg (0.85 M) of N-acetylglucosamine (acceptor) in 1 mL of green solvent (2 M)-buffer mixture was pre-equilibrated to 30 °C. Afterwards, 155 μmol/min (U) of β-galactosidase were added to the reaction mixture. Reaction was monitored by HPLC UV-vis and final products analysed by HPLC with an evaporative light scattering detector (ELSD). The reaction was stopped by heating the sample at 100 °C for 5 min.Isolation of disaccharides was done by carbon/Celite (50% m/m), chromatography, the column was eluted with milliQ water and ethanol gradient (from 5% to 15% v/v). and The structures of the disaccharides (Gal-β(1→4)GlcNAc and Gal-β(1→6)GlcNAc) were assigned by 1H and 13C NMR (D2O, 700 MHz), spectra were identical to previous references. , and In order to determine the effect of non-proteic substances present in Biolacta preparation in the reaction yield, transglycosylation reactions were carried out using semipurified (lyophilized) enzyme. The best performing solvents were chosen in view of the previous results, using crude extracts and setting the reactions with semipurified enzymes. The transglycosylation conditions were the same mentioned above and the reaction was monitored using HPLC-ELSD.For functionalized disaccharides the same protocol previously described was used, and different glycoconjugates as acceptors were added. A solution of 51.2 mg (0.17 M) pNP-β-Gal (donor) and the different monosaccharides functionalized 1 and 2 (0.51 M).86%With triethyl phosphate; sodium acetate; β-galactosidase In water at 30℃; galactosylation; Enzymatic reaction;
Safety and Hazards
GHS Hazard StatementsNot Classified
Source: European Chemicals Agency (ECHA)License Note: Use of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: “Source: European Chemicals Agency, http://echa.europa.eu/”. Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.License URL: https://echa.europa.eu/web/guest/legal-noticeRecord Name: (1-Cyano-2-ethoxy-2-oxoethylidenaminooxy)dimethylamino-morpholino-carbenium hexafluorophosphateURL: https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/213446Description: The information provided here is aggregated from the “Notified classification and labelling” from ECHA’s C&L Inventory. Read more: https://echa.europa.eu/information-on-chemicals/cl-inventory-database
Other Data
StorageStored at room temperature for long time; Sealed and keep away from light.
Use PatternN-Acetylglucosamine is a versatile ingredient widely used in pharmaceuticals, cosmetics, health supplements, and biomedical materials. Its primary sources include animal extraction and microbial fermentation.In the pharmaceutical field, it helps improve joint health, promotes cartilage matrix synthesis by chondrocytes, enhances cartilage repair, and alleviates joint pain. As a derivative of glucosamine, it supports skin health and tissue repair.In cosmetics, it stimulates the synthesis of hyaluronic acid (HA), enhancing skin hydration. It also repairs the skin barrier, reducing redness and sensitivity, while accelerating epidermal cell renewal and promoting wound healing. https://www.chemwhat.com/n-acetyl-glucosamine-cas-7512-17-6/
Comments
Post a Comment