IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
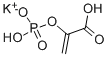
Product NamePHOSPHOENOLPYRUVIC ACID MONOPOTASSIUM SALTIUPAC Namepotassium;1-carboxyethenyl hydrogen phosphateMolecular StructureCAS Registry Number 4265-07-0MDL NumberMFCD00044476Synonyms4265-07-0Potassium 1-carboxyvinyl hydrogenphosphatePHOSPHOENOLPYRUVIC ACID MONOPOTASSIUM SALTPhospho(enol)pyruvic acid monopotassium saltMFCD00044476phosphoenolpyruvate potassium salt2-Propenoic acid, 2-(phosphonooxy)-, monopotassium saltpotassium;1-carboxyethenyl hydrogen phosphatePHOSPHOENOL PYRUVATE MONOPOTASSIUM SALTPEP-K;Potassium phosphoenolpyruvate;2-Phosphonooxy-acrylic acid potassium saltpotassium 2-(hydrogen phosphonooxy)prop-2-enoic acidPEP-KPotassium hydrogen 2-(phosphonatooxy)acrylateEINECS 224-247-0PhosphoenolpyruvicacidmonopotassiumsaltC3H4KO6PSCHEMBL2399312DTXSID80633812-Propenoic acid, 2-(phosphonooxy)-, potassium salt (1:1)AKOS016003884AKOS024307559Phosphoenolpyruvate, monopotassium saltGS-5446PD017213SY075611DB-050951Phosphoenol-pyruvate, PEP, monopotassium saltEN300-263193H10560Phospho(enol)pyruvic acid monopotassium salt, 99%potassium 2-(hydrogen phosphonatooxy)prop-2-enoic acidZ2429425999Phospho(enol)pyruvic acid monopotassium salt, >=97% (enzymatic)Molecular FormulaC3H4KO6PMolecular Weight206.13InChIInChI=1S/C3H5O6P.K/c1-2(3(4)5)9-10(6,7)8;/h1H2,(H,4,5)(H2,6,7,8);/q;+1/p-1InChI KeySOSDSEAIODNVPX-UHFFFAOYSA-MIsomeric SMILESC=C(C(=O)O)OP(=O)(O).
Physical Data
AppearanceWhite powder
Spectra
Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Temperature (NMR Spectroscopy), °C Spectrum1HChemical shifts31Pheavy water, water242.789Chemical shifts1HD2OChemical shifts31PH2O
Route of Synthesis (ROS)
Route of Synthesis (ROS) of PHOSPHOENOLPYRUVIC ACID MONOPOTASSIUM SALT CAS 4265-07-0
ConditionsYieldWith pKAD50; adenosine 5'-triphosphate sodium salt; Flavin mononucleotide; sodium dithionite; magnesium sulfate In sodium hydroxide at 23℃; for 1h; pH=8.1;67%
Safety and Hazards
Pictogram(s)SignalWarningGHS Hazard StatementsH315 (100%): Causes skin irritation H319 (100%): Causes serious eye irritation H335 (100%): May cause respiratory irritation Precautionary Statement CodesP261, P264, P264+P265, P271, P280, P302+P352, P304+P340, P305+P351+P338, P319, P321, P332+P317, P337+P317, P362+P364, P403+P233, P405, and P501(The corresponding statement to each P-code can be found at the GHS Classification page.)
Other Data
HS CodeStorageStore at 2~8°C for long time.Shelf Life1 yearMarket Price
DruglikenessLipinski rules componentMolecular Weight206.133logP-1.296HBA6HBD2Matching Lipinski Rules4Veber rules componentPolar Surface Area (PSA)116.7Rotatable Bond (RotB)3Matching Veber Rules2
Use PatternPhosphoenol pyruvate monopotassium salt is used as a substrate in enzyme reactions, particularly in glycolysis. It is the monopotassium salt form of phosphoenolpyruvate (PEP) and is commonly employed in studies related to enzymes involved in carbohydrate metabolism, such as pyruvate kinase (PK).
https://www.chemwhat.com/phosphoenolpyruvic-acid-monopotassium-salt-cas-4265-07-0/
Comments
Post a Comment