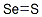
IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
Product NameSelenium sulfideMolecular StructureCAS Registry Number 7488-56-4EINECS Number231-303-8MDL NumberMFCD00MFCD00011227SynonymsSelenium disulfide7488-56-4Selenium disulphideSelenium(IV) disulfideSelenium(IV) sulfideSeledruffSelenium sulfide, micronizedSelenium sulphide, micronizedSelenium(IV) sulphideSelenium(IV) disulphideSelenium sulfide (USP)Selenium sulfide Selenium sulphide (ses2)Selsun BlueSelenium sulfide (ses(sub 2))Z69D9E381QSebusanSelenexSelenixSelukosLeniumSeleenSulfur selenideZeranSul-BlueSel-O-RinseSelenium sulfide (SeS2)Selen(IV) sulfidSelenium sulfide SeS2Caswell No. 732ASelendisulfidTersiSelenii disulfidumHSDB 6367Selenium(IV) Disulfide (1:2)EINECS 231-303-8UN2657RCRA waste no. U205EPA Pesticide Chemical Code 072003UNII-Z69D9E381QExsel (TN)SELENIUM DISULFIDE SELENIUM SULFIDE CHEMBL1200680DTXSID0042375SELENIUM SULFIDE SELENIUM DISULFIDE SELENIUM SULFIDE CHEBI:135934JNMWHTHYDQTDQZ-UHFFFAOYSA-NSELENIUM SULFIDE SELENIUM SULFIDE SELENIUM DISULFIDE SELENIUM SULFIDE DB00971SELENIUM DISULFIDE SELENIUM SULFIDE SELENIUM DISULFIDE SELENII DISULFIDUM Selenium disulfide NS00078792D05815Q56249646Molecular FormulaS2SeMolecular Weight143.1InChIInChI=1S/S2Se/c1-3-2InChI KeyJNMWHTHYDQTDQZ-UHFFFAOYSA-NIsomeric SMILESS==S
Physical Data
AppearanceRed powder
Spectra
Description (IR Spectroscopy)Spectrum
Route of Synthesis (ROS)
Route of Synthesis (ROS) of Selenium sulfide CAS# 7488-56-4
ConditionsYieldIn further solvent(s) soln. of SeCl4 in liquid H2S, thiohydrolysis;; formation of sulfide;;In further solvent(s) soln. of SeCl4 in liquid H2S, thiohydrolysis;; formation of sulfide;In further solvent(s) soln. of SeCl4 in liquid H2S, thiohydrolysis;; formation of sulfide;
Safety and Hazards
Pictogram(s)SignalDangerGHS Hazard StatementsH300 (10.09%): Fatal if swallowed H301+H331 (33.94%): Toxic if swallowed or if inhaled H301 (86.24%): Toxic if swallowed H330 (11.93%): Fatal if inhaled H331 (84.4%): Toxic if inhaled H373 (100%): May causes damage to organs through prolonged or repeated exposure H400 (94.5%): Very toxic to aquatic life H410 (93.58%): Very toxic to aquatic life with long lasting effects Precautionary Statement CodesP260, P261, P264, P270, P271, P273, P284, P301+P316, P304+P340, P316, P319, P320, P321, P330, P391, P403+P233, P405, and P501(The corresponding statement to each P-code can be found at the GHS Classification page.)
Other Data
TransportationUnder the room temperature and away from lightHS CodeStorageUnder the room temperature and away from lightShelf Life1 yearMarket Price
DruglikenessLipinski rules componentMolecular Weight143.092logP-0.296HBA0HBD0Matching Lipinski Rules4Veber rules componentPolar Surface Area (PSA)64.18Rotatable Bond (RotB)0Matching Veber Rules2
Use PatternSelenium disulfide, also known as selenium sulfide, is a chemical compound with the formula SeS2, where selenium and sulfur are combined in a 1:2 ratio. It is best known for its application in medical and personal care products, particularly as an active ingredient in anti-dandruff shampoos and as a treatment for certain skin conditions. TSelenium disulfide's unique properties and its role in health and skincare highlight its importance in both medical dermatology and cosmetic formulations.The primary mechanism through which selenium disulfide exerts its effects is by reducing the proliferation of Malassezia, a genus of fungi that naturally resides on the skin. Overgrowth of these fungi can lead to dandruff and seborrheic dermatitis, conditions characterized by flaky and itchy scalp. Selenium disulfide's antifungal properties help regulate the presence of these fungi, thereby controlling the symptoms associated with their overgrowth.Apart from its antifungal action, Selenium disulfide is also known for its keratolytic activity, meaning it can help in removing dead skin cells and reducing scaliness. This makes it effective not only against dandruff but also in the treatment of tinea versicolor, a fungal infection that affects the skin's pigmentation, resulting in discolored patches.In the formulation of anti-dandruff shampoos and lotions, the concentration of selenium disulfide is carefully regulated. Typically, over-the-counter products contain it at concentrations ranging from 1% to 2.5%, ensuring effectiveness while minimizing potential side effects. These may include skin irritation, oiliness or dryness of the scalp and hair, and a temporary change in hair color. Despite these side effects, selenium disulfide is considered safe for most users when used as directed.The use of selenium disulfide extends beyond personal care products; it also has implications in various industrial applications. However, its most significant impact remains in the field of dermatology, where it provides a critical solution for managing dandruff, seborrheic dermatitis, and tinea versicolor.Ongoing research into selenium disulfide aims to further understand its mechanisms of action, potential resistance development by target fungi, and ways to enhance its efficacy and reduce side effects. As science advances, new formulations and applications of selenium disulfide may emerge, expanding its role in healthcare and personal care industries.In summary, selenium disulfide is a vital compound in the fight against dandruff and certain skin conditions, offering relief to millions of people worldwide. Its continued study and application promise to improve the quality of life for those affected by these common yet troublesome conditions.
https://www.chemwhat.com/selenium-sulfide-cas-7488-56-4/
Comments
Post a Comment