IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
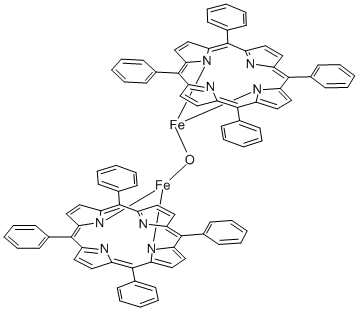
Product NameIRON (III) MESO-TETRAPHENYLPORPHINE-MU-OXO DIMERIUPAC Nameiron(2+);5,10,15,20-tetraphenylporphyrin-22,24-diide;hydrateMolecular StructureCAS Registry Number 12582-61-5Synonyms12582-61-5IRON (III) MESO-TETRAPHENYLPORPHINE-MU-OXO DIMERIron(III) meso-tetraphenylporphine-mu-oxo dimerMeso-5,10,15,20-Tetraphenyl-21H,23H-porphineironu-oxodimeriron(2+);5,10,15,20-tetraphenylporphyrin-22,24-diide;hydrateMFCD00058898AKOS015911269Iron,m-oxobisdi-2,7,12,17-tetraphenyl-22-({2,7,12,17-tetraphenyl-21,23,24,25-tetraaza-22-ferrahexacyclopentacosa-1,3(25),4,6,8,10,12,14,16(24),17,19-undecaen-22-yl}oxy)-21,23,24,25-tetraaza-22-ferrahexacyclopentacosa-1,3(25),4,6,8,10,12,14,16(24),17,19-undecaeneMolecular FormulaC88H58Fe2N8OMolecular Weight1355.1InChIInChI=1S/2C44H28N4.2Fe.H2O/c2*1-5-13-29(14-6-1)41-33-21-23-35(45-33)42(30-15-7-2-8-16-30)37-25-27-39(47-37)44(32-19-11-4-12-20-32)40-28-26-38(48-40)43(31-17-9-3-10-18-31)36-24-22-34(41)46-36;;;/h2*1-28H;;;1H2/q2*-2;2*+2InChI KeyJTFKJCUSDKAMIA-UHFFFAOYSA-NIsomeric SMILESC1=CC=C(C=C1)C2=C3C=CC(=C(C4=NC(=C(C5=CC=C(5)C(=C6C=CC2=N6)C7=CC=CC=C7)C8=CC=CC=C8)C=C4)C9=CC=CC=C9)3.C1=CC=C(C=C1)C2=C3C=CC(=C(C4=NC(=C(C5=CC=C(5)C(=C6C=CC2=N6)C7=CC=CC=C7)C8=CC=CC=C8)C=C4)C9=CC=CC=C9)3.O..
Physical Data
AppearancePurple powder
Spectra
Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Temperature (NMR Spectroscopy), °C Frequency (NMR Spectroscopy), MHzChemical shifts, Spectrum1Hchloroform-d14001Hdichloromethane-d21HC6D5CD3=toluene-d825
Description (IR Spectroscopy)Solvent (IR Spectroscopy)BandsKBrBandsnot given
Description (UV/VIS Spectroscopy)Solvent (UV/VIS Spectroscopy)Comment (UV/VIS Spectroscopy)Absorption Maxima (UV/VIS), nmSpectrum, Band assignmentmethylene chloride=methylene dichloride340 nm - 760 nm258Band assignmentnot given410Spectrummethylene chloride=methylene dichloride250 nm - 2000 nmpyridine410 nm - 614 nmdichloroethane=1,2-dichloroethane407 nm - 613 nmSpectrumbenzene450 nm - 710 nm
Route of Synthesis (ROS)
Route of Synthesis (ROS) of IRON (III) MESO-TETRAPHENYLPORPHINE-MU-OXO DIMER CAS# 12582-61-5
ConditionsYieldIn dichloromethane for 0.75h; Inert atmosphere;Experimental Procedure(TPP)Fe(OC(=O)CCl3 (1)To a CH2Cl2 solution (15 mL) of 2(μ-O) (0.033 g, 0.024 mmol) was added trichloroacetic acid (0.010 g, 0.06 mmol). The mixture was stirred for 45 min, during which time the color of the solution changed from green to brown. The solvent was reduced to ∼3 mL and hexane (10 mL) was added. The solution was slowly concentrated under reduced pressure until precipitation of the product occurred. The dark brown precipitate was collected by filtration, washed with hexane (2 × 15 mL), and dried in vacuo to give (TPP)Fe(OC(=O)CCl3) (0.020 g, 0.024 mmol, 50% isolated yield). Slow evaporation of a CH2Cl2/cyclohexane (1:1 ratio; 4 mL) solution of the product at room temperature gave suitable crystals for X-ray diffraction studies. IR (CH2Cl2, cm-1): υOCO(asym) = 1704. IR (KBr, cm-1): υOCO(asym) = 1709. UV-vis (λ (relative ε, mM-1cm-1), 2 × 10-5 M in CH2Cl2): 411 (138), 509 (14), 571 (8), 681 (4) nm.50%In benzene Kinetics; byproducts: H2O; Fe-complex dimer thermostatization at 298.15+/-0.05 K, trichloracetic acid addn.; monitoring by UV/VIS spectroscopy;
Safety and Hazards
GHS Hazard StatementsNot Classified
Other Data
TransportationUnder the room temperature and away from lightHS CodeStorageUnder the room temperature and away from lightShelf Life1 yearMarket Price
DruglikenessLipinski rules componentMolecular Weight1353.16logPHBA9HBD0Matching Lipinski Rules2Veber rules componentPolar Surface Area (PSA)9.23Rotatable Bond (RotB)10Matching Veber Rules2
Use PatternIron(III) meso-tetraphenylporphine-mu-oxo dimer CAS 12582-61-5 are widely used to catalyze the oxidation reactions of organic molecules.
https://www.chemwhat.com/iron-iii-meso-tetraphenylporphine-mu-oxo-dimer-cas-12582-61-5/
Comments
Post a Comment