IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
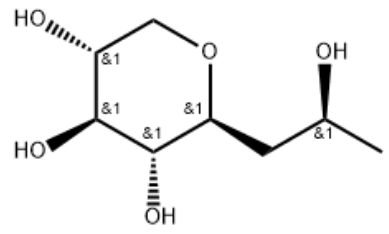
Product Name(S)-Pro-xylaneIUPAC NameMolecular Structure CAS Registry Number 868156-46-1EINECS NumberMDL NumberMFCD32701904Beilstein Registry NumberSynonymsMolecular FormulaC8H16O5Molecular Weight192.210InChIInChI KeyCanonical SMILES
Patent InformationPatent IDTitlePublication DateUS2005/250708Novel C-glycosides, uses thereof2005
Physical Data
AppearanceSolid
Melting Point, °C 120 - 122
Spectra
Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Chemical shifts, Spectrum1Hwater-d2Chemical shifts, Spectrum13Cwater-d2Chemical shifts1HChemical shifts13CChemical shifts1HCD3OD
Route of Synthesis (ROS)
Route of Synthesis (ROS) of (S)-Pro-xylane CAS# 868156-46-1
ConditionsYieldWith sodium tris(acetoxy)borohydride In isopropyl alcohol at 20℃; Product distribution / selectivity;Experimental Procedure2 Production of C-β-D-xylopyranoside-2-(S)-hydroxypropaneEXAMPLE 2 Production of C-β-D-xylopyranoside-2-(S)-hydroxypropane The expected compound was obtained according the process described in Example 1, replacing the 2 ml of acetic acid and the sodium borohydride with two times 1.2 equivalents of sodium triacetoxyborohydride (NaBH(OAc)3). The product was obtained with a quantitative yield, and selectively in (β, S) form. The physico chemical characteristics are identical in all respects to those obtained in Example 1.100%With sodium tetrahydroborate; acetic acid In isopropyl alcohol at 20℃; for 1.5h; Product distribution / selectivity;Experimental Procedure1 Production of C-β-D-xylopyranoside-2-(S)-hydroxypropaneEXAMPLE 1 Production of C-β-D-xylopyranoside-2-(S)-hydroxypropane 2 ml of acetic acid, followed rapidly by 120 mg (1.2 eq.) of sodium borohydride as granules were added to a solution of 500 mg of C-β-D-xylopyranoside-2-propanone (described in Example 1 of application WO-02/051828) in 9 ml of isopropanol. The medium was left at ambient temperature for 30 minutes with stirring. 120 mg (1.2 eq.) of sodium borohydride as granules were then added. The reaction medium was left for 1 hour at ambient temperature with stirring. 10 ml of acetone were then added and, after stirring for 30 minutes at ambient temperature, the reaction medium was concentrated under vacuum. The residue obtained was purified by silica gel chromatography so as to selectively produce the expected compound C-β-D-xylopyranoside-2-(S)-hydroxypropane with a 95% yield. Physico chemical characteristics of the compound: Melting point: 120-122° C. Optical rotation: -37° (at 20° C. in methanol, and at a concentration =1 g/100 ml) 1H NMR: 1.03 (t, 3H); 1.46 (m, 1H); 1.71 (m, 1H); 2.85 (m, 1H); 2.94 (m, 1H); 2.99 (m, 2H); 3.24 (m, 1H); 3.67 (m, 1H); 3.77 (m, 1H) Structure confirmed by X-ray diffraction.95%
Safety and Hazards
Pictogram(s)SignalGHS Hazard StatementsNot ClassifiedPrecautionary Statement Codes
Other Data
DruglikenessLipinski rules componentMolecular Weight192.212logP-1.202HBA5HBD4Matching Lipinski Rules4Veber rules componentPolar Surface Area (PSA)90.15Rotatable Bond (RotB)2Matching Veber Rules2
Use PatternAnti-aging, promotes skin cell renewal, helps repair and rebuild the skin's collagen and glycosaminoglycans, thereby reducing wrinkles and fine lines. Moisturizing and nourishing, promotes the synthesis of collagen, repairs damaged skin; enhances overall skin health.
https://www.chemwhat.com/s-pro-xylane-cas-868156-46-1/
Comments
Post a Comment