IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
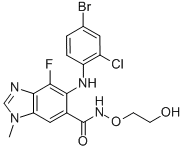
Product NameSelumetinibIUPAC Name6-(4-bromo-2-chloroanilino)-7-fluoro-N-(2-hydroxyethoxy)-3-methylbenzimidazole-5-carboxamideMolecular StructureCAS Registry Number 606143-52-6EINECS Number207-313-3MDL NumberMFCD11977472SynonymsSelumetinib606143-52-6AZD6244ARRY-142886AZD 62445--4-FLUORO-N-(2-HYDROXYETHOXY)-1-METHYL-1H-BENZIMIDAZOLE-6-CARBOXAMIDEAZD-6244ARRY 142886Selumetinib (AZD6244)ARRY-886selumetinibumUNII-6UH91I579U5-((4-bromo-2-chlorophenyl)amino)-4-fluoro-N-(2-hydroxyethoxy)-1-methyl-1H-benzoimidazole-6-carboxamide6UH91I579UARRY142886Selumetinib KoselugoDTXSID3048944CHEBI:902275-(4-broMo-2-chlorophenylaMino)-4-fluoro-N-(2-hydroxyethoxy)-1-Methyl-1H-benzoiMidazole-6-carboxaMideCHEMBL1614701DTXCID0028870NSC 741O78NSC741078NCGC00189073-01NCGC00189073-021H-Benzimidazole-6-carboxamide, 5-((4-bromo-2-chlorophenyl)amino)-4-fluoro-N-(2-hydroxyethoxy)-1-methyl-1H-Benzimidazole-6-carboxamide, 5--4-fluoro-N-(2-hydroxyethoxy)-1-methyl-AZD6244 (Selumetinib)6-(4-bromo-2-chloroanilino)-7-fluoro-N-(2-hydroxyethoxy)-3-methylbenzimidazole-5-carboxamide5--4-fluoro-N-(2-hydroxyethoxy)-1-methyl-1H-1,3-benzodiazole-6-carboxamide6--7-fluoro-N-(2-hydroxyethoxy)-3-methylbenzimidazole-5-carboxamideAZD 6244;5-((4-Bromo-2-chlorophenyl)amino)-4-fluoro-N-(2-hydroxyethoxy)-1-methyl-1H-benzoimidazole-6-carboxamide;6-(4-bromo-2-chlorophenylamino)-7-fluoro-N-(2-hydroxyethoxy)-3-methyl-3H-benzoimidazole-5-carboxamideCAS-606143-52-6C17H15BrClFN4O33EW5-((4-BROMO-2-CHLOROPHENYL)AMINO)-4-FLUORO-N-(2-HYDROXYETHOXY)-1-METHYL-1H-BENZIMIDAZOLE-6-CARBOXAMIDESELUMETINIB SELUMETINIB Selumetinib (USAN/INN)SELUMETINIB MEK inhibitor AZD6244SELUMETINIB SCHEMBL155456GTPL5665EX-A020CYOHGALHFOKKQC-UHFFFAOYSA-NBCPP000367HMS3244G03HMS3244G04HMS3265K01HMS3265K02BCP01739Tox21_113362BDBM50355497MFCD11977472NSC800882s1008AKOS015904255Tox21_113362_1BCP9000354CCG-264774CS-0059DB11689EX-8621NSC-741078NSC-800882SB14707NCGC00189073-076-(4-bromo-2-chloro-anilino)-7-fluoro-N-(2-hydroxyethoxy)-3-methyl-benzimidazole-5-carboxamideAC-25059AM808016AZD6244,Selumetinib, ARRY-142886HY-50706Selumetinib (ARRY142886/AZD6244)AZD6244 (Selumetinib,ARRY-142886)A8446NS00071922SW202561-3D09666EN300-18166787Q-101405Q7448840BRD-K57080016-001-01-91H-BENZIMIDAZOLE-6-CARBOXAMIDE, 5-((4-BROMO-2-CHLOROPHENYL)AMINO)-4-FLUORO-N-(2- HYDROXYETHOXY)-1-METHYL-6-(4-bromo-2-chloro-phenylamino)-7-fluoro-3-methyl-3H-benzoimidazole-5-carboxylic acid (2-hydroxy -ethoxy)-amide6-(4-Bromo-2-chloro-phenylamino)-7-fluoro-3-methyl-3H-benzoimidazole-5-carboxylic acid (2-hydroxy-ethoxy)-amide6-(4-bromo-2-chloro-phenylamino)-7-fluoro-3-methyl-3H-benzoimidazole-5-carboxylic acid(2-hydroxy-ethoxy)-amide6-(4-bromo-2-chlorophenylamino)-7-fluoro-3-methyl-3H-benzoimidazole-5-carboxylic acid (2-hydroxyethoxy)-amide6-(4-bromo-2-chlorophenylamino)-7-fluoro-3-methyl-3H-benzoimidazole-5-carboxylic acid(2-hydroxyethoxy)-amide6-(4-bromo-2chloro-phenylamino)-7-fluoro-3-methyl-3H-benzoimidazole-5-carboxylic acid (2-hydroxy-ethoxy)-amideMolecular FormulaC17H15BrClFN4O3 Molecular Weight457.7InChIInChI=1S/C17H15BrClFN4O3/c1-24-8-21-16-13(24)7-10(17(26)23-27-5-4-25)15(14(16)20)22-12-3-2-9(18)6-11(12)19/h2-3,6-8,22,25H,4-5H2,1H3,(H,23,26)InChI KeyCYOHGALHFOKKQC-UHFFFAOYSA-NIsomeric SMILESCCN1C=NC2=C1C=C(C(=C2F)NC3=C(C=C(C=C3)Br)Cl)C(=O)NOCCO
Patent InformationPatent IDTitlePublication DateCN109438362Substituted benzimidazole compound and composition with compound2019WO2018/65924INTERMEDIATES OF MITOGEN-ACTIVATED PROTEIN KINASE KINASE (MAP2K OR MEK) INHIBITORS AND PROCESS FOR THEIR PREPARATION2018US2004/116710N3 alkylated benzimidazole derivatives as MEK inhibitors2004US2003/232869N3 alkylated benzimidazole derivatives as MEK inhibitors2003
Physical Data
AppearanceWhite or off-white powder.
Spectra
Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Frequency (NMR Spectroscopy), MHzSolid state NMR, Chemical shifts, Spectrum19F3761HCD3OD40019FCD3OD3761HCD3OD
Route of Synthesis (ROS)
Route of Synthesis (ROS) of Selumetinib CAS# 606143-52-6
ConditionsYieldWith hydrogenchloride; water In ethanol for 24h;Experimental ProcedureHydrochloric acid (14 mL, 1.0 M aqueous solution, 14 mmol) was added to a suspension of 6-(4-bromo-2-chloro- phenylamino)-7-fluoro-3-methyl-3H-benzoimidazole-5-carboyxlic acid (2-vinyloxyethoxy)- amide (2.18 g, 4.50 mmol) in ethanol (50 mL) and the reaction mixture allowed to stir for 24 hours. The reaction mixture was concentrated to dryness by rotary evaporation and the solids partitioned between 3:1 ethyl acetate/tetrahydrofuran and saturated potassium carbonate. The aqueous phase was extracted with 3:1 ethyl acetate/tetrahydrofuran (3x), the combined organics dried (Na2SO4), and concentrated to provide 2.11 g (100%) 6-(4-bromo-2- chlorophenylamino)-7-fluoro-3-methyl-3H-benzoimidazole-5-carboxylic acid (2- hydroxyethoxy)-amide as an off-white solid. MS ESI (+) m/z 457, 459 (M+, Br pattern) detected. 1H NMR (400 MHz, MeOH-&0 δ 8.26 (s, IH), 7.78 (s, IH), 7.57 (d, IH), 7.24 (dd, IH), 6.40 (dd, IH), 3.86 (s, 3H), 3.79 (m, 2H), 3.49 (m, 2H). 19F NMR (376 MHz, MeOH-d4) -133.68 (s).100%With hydrogenchloride In ethanol; water at 25 - 30℃; for 24h;Experimental Procedure7 Example 7: Preparation of 5-H318 (100%): Causes serious eye damage H361 (100%): Suspected of damaging fertility or the unborn child H373 (100%): May causes damage to organs through prolonged or repeated exposure H411 (100%): Toxic to aquatic life with long lasting effects Precautionary Statement CodesP203, P260, P261, P264+P265, P272, P273, P280, P302+P352, P305+P354+P338, P317, P318, P319, P321, P333+P317, P362+P364, P391, P405, and P501(The corresponding statement to each P-code can be found at the GHS Classification page.)
Other Data
TransportationHS CodeStorageUnder the room temperature and away from lightShelf Life2 yearsMarket Price
DruglikenessLipinski rules componentMolecular Weight457.687logP3.696HBA5HBD3Matching Lipinski Rules4Veber rules componentPolar Surface Area (PSA)88.41Rotatable Bond (RotB)7Matching Veber Rules2
Use Pattern Selumetinib CAS#: 606143-52-6 is used for the treatment of advanced non-small cell lung cancer (NSCLC). Selumetinib primarily inhibits the growth of various tumors, including melanomas with B-Raf mutations and non-small cell lung cancer (NSCLC) with K-Ras mutations, by regulating the key protein kinase MEK in the Ras-Raf-MEK-ERK pathway. It is mainly employed in the treatment of diseases such as cholangiocarcinoma, colorectal cancer, and NSCLC. Currently, Selumetinib is in phase III clinical trials for the treatment of non-small cell lung cancer.
https://www.chemwhat.com/selumetinib-cas-606143-52-6/
Comments
Post a Comment