IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
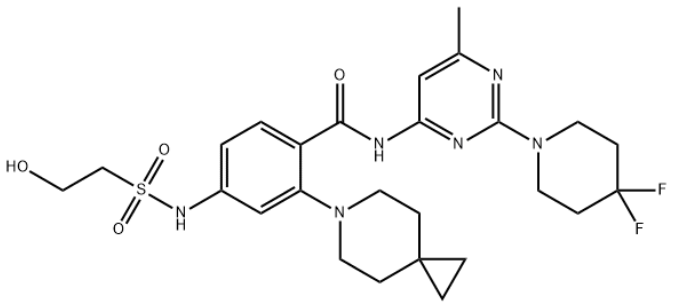
Product NameAMG-650IUPAC Namepyridin-3-amineMolecular StructureCAS Registry Number 2410796-79-9SynonymsSovilnesib2410796-79-9AMG-650AMG 650 OP393M4H32UNII-OP393M4H32AMG 650Benzamide, 2-(6-azaspiro(2.5)oct-6-yl)-N-(2-(4,4-difluoro-1-piperidinyl)-6-methyl-4-pyrimidinyl)-4-(((2-hydroxyethyl)sulfonyl)amino)-Sovilnesib(AMG-650)?CHEMBL5084301SCHEMBL22119383KBDGEJDIHZDPHN-UHFFFAOYSA-NUS11236069, Example 4BDBM535532EX-A6760US11236069, Example 15MFCD34578308NSC839866AKOS040757673NSC-839866AC-37121MS-30230N--4-(2-hydroxyethylsulfonamido)-2-(6-azaspirooctan-6-yl)benzamideSY345506HY-132840CS-0204143G185092-(6-azaspirooctan-6-yl)-N--4-(2-hydroxyethylsulfonylamino)benzamideN-(2-(4,4-Difluoropiperidin-1-yl)-6- methylpyrimidin-4-yl)-4-((2- hydroxyethyl)sulfonamido)-2-(6- azaspirooctan-6-yl)benzamideMolecular FormulaC26H34F2N6O4SMolecular Weight564.6InChIInChI=1S/C26H34F2N6O4S/c1-18-16-22(31-24(29-18)34-12-8-26(27,28)9-13-34)30-23(36)20-3-2-19(32-39(37,38)15-14-35)17-21(20)33-10-6-25(4-5-25)7-11-33/h2-3,16-17,32,35H,4-15H2,1H3,(H,29,30,31,36)InChI KeyKBDGEJDIHZDPHN-UHFFFAOYSA-NIsomeric SMILESCC1=CC(=NC(=N1)N2CCC(CC2)(F)F)NC(=O)C3=C(C=C(C=C3)NS(=O)(=O)CCO)N4CCC5(CC5)CC4
Physical Data
AppearanceSolid
Spectra
No data available
Route of Synthesis (ROS)
No data available
Safety and Hazards
GHS Hazard StatementsNot Classified
Other Data
HS CodeStorageStore at -20°C and away from lightShelf Life2 yearsMarket Price
Use PatternAMG 650 is a highly efficient and selective inhibitor targeting KIF18A, an overexpressed kinesin motor protein in human cancers. It selectively inhibits KIF18A MT-ATPase motor activity, displaying specificity towards various kinesin proteins. At concentrations affecting sensitive tumor cells, AMG 650 has minimal impact on the in vitro proliferation of human bone marrow mononuclear cells (>100 times). In vivo, AMG 650 exhibits low clearance, a long half-life, and favorable oral bioavailability. Additionally, when combined with the PARP inhibitor olaparib, AMG 650 enhances anticancer activity in tumor models with alterations in BRCA1 and CCNE1 compared to single-agent treatment.
https://www.chemwhat.com/amg-650-cas-2410796-79-9/
Comments
Post a Comment