IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
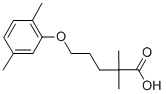
Product NameGemfibrozilIUPAC Name5-(2,5-dimethylphenoxy)-2,2-dimethylpentanoic acidMolecular StructureCAS Registry Number 25812-30-0EINECS Number247-280-2MDL NumberMFCD00079335Synonymsgemfibrozil25812-30-05-(2,5-Dimethylphenoxy)-2,2-dimethylpentanoic acidLopidJezilDecrelipLipurCI-719CholespidFibratolFibrocitGemfibrilGemfibromaxGemlipidHipolixanGemfibroziloGemfibrozilumRenabrazinClearolElmoganFetinorGemnpidInnogenIpolipidLanateromLifibronLipigemLipizylMicolipNormolipProgemzalReducelRegulipSinelipSynbrozilTaborcilTentrocBrozilGozidHidilLipiraGemdGevilon UnoWL-GemfibrozilGen-FibroPentanoic acid, 5-(2,5-dimethylphenoxy)-2,2-dimethyl-Low-LipGem-SLipozid2,2-Dimethyl-5-(2,5-xylyloxy)valeric acid2,2-Dimethyl-5-(2,5-xylyloxy)valeriansaeureGemfibrozilum Gemfibrozilo CCRIS 3182,2-Dimethyl-5-(2,5-dimethylphenoxy)valeriansaeureEINECS 247-280-2NSC-757024BRN 1881200Gemfibrozil (Lopid)Valeric acid, 2,2-dimethyl-5-(2,5-xylyloxy)-BolutolCHEBI:5296Apo-GemfibrozilDTXSID0020652UNII-Q8X02027X3HSDB 7735Gemfibrozil (Standard)GemfibrosilLipazilLitarekMFCD00079335TrialminAusgemPilderCHEMBL457Q8X02027X3DTXCID20652MLS000028421Gemfibrozil 2,2-Dimethyl-5-(2,5-dimethylphenoxy)pentanoic acid5-(2,5-dimethylphenoxy)-2,2-dimethyl-pentanoic acidNSC 7570245--2,2-dimethylpentanoic acidNCGC00016794-09SMR000058393GenlipCAS-25812-30-0GEMFIBROZIL (IARC)GEMFIBROZIL Gemfibrozilum (INN-Latin)Gemfibrozilo (INN-Spanish)GEMFIBROZIL (MART.)GEMFIBROZIL GEMFIBROZIL (USP-RS)GEMFIBROZIL 2,2-DIMETHYL-5-(2,5-XYLYLOXY) VALERIC ACIDGEMFIBROZIL (EP MONOGRAPH)GEMFIBROZIL InnogemGemcorGEMFIBROZIL (USP MONOGRAPH)GEMFIBROZIL Gemfibrozil (USAN:USP:INN:BAN)CI 719Lopid (TN)TEVA-ASR-01000000056Gemfibrozil (JAN/USP/INN)Gemfibrozil,(S)4TXPrestwick_637dimethylpentanoic acidSpectrum_000825CPD000058393GEMFIBROZIL 114413-98-8Opera_ID_1658Prestwick0_000214Prestwick1_000214Prestwick2_000214Prestwick3_000214Spectrum2_001097Spectrum3_000440Spectrum4_000562Spectrum5_000750Spectrum5_001991GEMFIBROZIL GEMFIBROZIL GEMFIBROZIL GEMFIBROZIL GEMFIBROZIL GEMFIBROZIL SCHEMBL4813BSPBio_000227BSPBio_002060GEMFIBROZIL KBioGR_000964KBioSS_001305MLS001055364MLS006011850DivK1c_000138SPECTRUM1500313SPBio_001174SPBio_002148BPBio1_000251GTPL3439YSSJ5501Gemfibrozil, analytical standardCl-719HMS500G20HY-B0258RKBio1_000138KBio2_001305KBio2_003873KBio2_006441KBio3_001280C10AB04GEMFIBROZIL Gemfibrozil for system suitabilityNINDS_000138HMS1568L09HMS1920B07HMS2090K14HMS2091H11HMS2095L09HMS2230H24HMS3259M12HMS3655C06HMS3712L09Pharmakon1600-105003135-(2,5-dimethylphenoxy)-2,2-BCP08437HY-B0258Tox21_110613Tox21_201997Tox21_302784BBL010807BDBM50110590CCG-40111DL-414NSC757024s1729STK618740AKOS001606691Tox21_110613_1AB03034AC-4225DB01241KS-5192MCULE-4563546953NC00565IDI1_000138NCGC00016794-01NCGC00016794-02NCGC00016794-03NCGC00016794-04NCGC00016794-05NCGC00016794-06NCGC00016794-07NCGC00016794-08NCGC00016794-10NCGC00016794-11NCGC00016794-13NCGC00016794-14NCGC00022722-03NCGC00022722-04NCGC00022722-05NCGC00022722-06NCGC00022722-07NCGC00256601-01NCGC00259546-01SY052512Gemfibrozil 100 microg/mL in AcetonitrileSBI-0051391.P0032,2-Dimethyl-5-(2,5-zlyloxy)valeric acidAB00052003CS-0694895G0368NS00010281SW196802-32,2-Dimethyl-5-(2,5-xylyloxy)valeriansaureEN300-97098C07020D00334D83091AB00052003-15AB00052003-16AB00052003_17AB00052003_18A818037Q3842952,2-Dimethyl-5-(2,5-dimethylphenoxy)valeriansaure2,2-Dimethyl-5-(2,5-dimethylphenoxy)valeric Acid5-(2,5-dimethyphenoxy)-2,2-dimethylpentanoic acidSR-01000000056-3SR-01000000056-4SR-01000000056-6SR-01000000056-7W-1072162,2-Dimethyl-5-(2,5-dimethylphenoxy)-pentanoic acidBRD-K11129031-001-05-1Z1259021151Gemfibrozil, British Pharmacopoeia (BP) Reference StandardGemfibrozil, European Pharmacopoeia (EP) Reference StandardGemfibrozil, United States Pharmacopeia (USP) Reference StandardGemfibrozil, Pharmaceutical Secondary Standard; Certified Reference MaterialGemfibrozil for system suitability, European Pharmacopoeia (EP) Reference Standard2,2-Dimethyl-5-(2,5-dimethylphenoxy)pentanoic acid, 2,2-Dimethyl-5-(2,5-xylyloxy)valeric acid, 5-(2,5-Dimethylphenoxy)-2,2-dimethylpentanoic aciMolecular FormulaC15H22O3Molecular Weight250.33InChIInChI=1S/C15H22O3/c1-11-6-7-12(2)13(10-11)18-9-5-8-15(3,4)14(16)17/h6-7,10H,5,8-9H2,1-4H3,(H,16,17)InChI KeyHEMJJKBWTPKOJG-UHFFFAOYSA-NIsomeric SMILESCC1=CC(=C(C=C1)C)OCCCC(C)(C)C(=O)O
Patent InformationPatent IDTitlePublication DateWO2023/155912RECYCLABLE HIGH-REACTIVITY HYPERVALENT IODINE REAGENT2023CN114149341Aryloxycyclohexyl amide AMPK agonist as well as preparation method and medical application thereof2021CN112573978Efficient halogenation synthesis method of aryl halide2021WO2020/128816PHARMACEUTICAL COMPOSITIONS AND METHODS COMPRISING A COMBINATION OF A BENZOXAZOLE TRANSTHYRETIN STABILIZER AND AN ADDITIONAL THERAPEUTIC AGENT2020WO2020/243754LIGAND-ENABLED ß-C(sp3)–H LACTONIZATION FOR ß-C–H FUNCTIONALIZATIONS2020
Physical Data
AppearanceWhite crystalline powder
Melting Point, °C 33461.259 - 6158 - 7061 - 63
Boiling Point, °C158 - 159
Density, g·cm-31.09
Description (Association (MCS))Solvent (Association (MCS))Partner (Association (MCS))Stability constant of the complex with ...various solvent(s)human serum albuminStability constant of the complex with ...various solvent(s)immobilized phospholipid membraneFurther physical properties of the complexH2OLixAl2(OH)6*yH2O
Spectra
Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Frequency (NMR Spectroscopy), MHzChemical shifts, Spectrum13Cchloroform-d1Chemical shifts, Spectrum1Hchloroform-d1500Chemical shifts, Spectrum13Cchloroform-d1126Spectrum1HChemical shifts, Spectrum1Hchloroform-d1400
Description (IR Spectroscopy)Solvent (IR Spectroscopy)Bands, SpectrumSpectrumBandspotassium bromideBandsBands, Spectrumpotassium bromide
Description (UV/VIS Spectroscopy)Solvent (UV/VIS Spectroscopy)Comment (UV/VIS Spectroscopy)Absorption Maxima (UV/VIS), nmSpectrum198.3, 275.2Spectrumwater, dimethyl sulfoxide274Spectrummethanol, water
Route of Synthesis (ROS)
Route of Synthesis (ROS) of Gemfibrozil CAS# 25812-30-0
ConditionsYieldWith dmap; dicyclohexyl-carbodiimide In dichloromethane98%With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In tetrahydrofuran at 20℃; Inert atmosphere;98%With dmap; dicyclohexyl-carbodiimide In dichloromethane at 20℃;94%
Safety and Hazards
Pictogram(s)SignalWarningGHS Hazard StatementsH302 (100%): Harmful if swallowed H351 (24.62%): Suspected of causing cancer H361 (26.15%): Suspected of damaging fertility or the unborn child H411 (24.62%): Toxic to aquatic life with long lasting effects Precautionary Statement CodesP203, P264, P270, P273, P280, P301+P317, P318, P330, P391, P405, and P501(The corresponding statement to each P-code can be found at the GHS Classification page.)
Other Data
TransportationUnder the room temperature and away from lightHS CodeStorageUnder the room temperature and away from lightShelf Life3 yearsMarket Price
DruglikenessLipinski rules componentMolecular Weight250.338logP3.35HBA2HBD1Matching Lipinski Rules4Veber rules componentPolar Surface Area (PSA)46.53Rotatable Bond (RotB)6Matching Veber Rules2
Use PatternGemfibrozil, with the chemical name 2,2-dimethyl-5-(2,5-dimethylphenoxy)pentanoic acid. Gemfibrozil is a fibrate class lipid-regulating medication derived from clofibrate. It was introduced to the market in the United States in 1982. Gemfibrozil overcomes the serious hepatic toxicity associated with earlier lipid-lowering drugs such as clofibrate while retaining its efficacy. It promotes the breakdown of peripheral fat, reduces the hepatic uptake of free fatty acids, thereby decreasing intrahepatic triglyceride formation. Additionally, it inhibits the synthesis of very-low-density lipoprotein (VLDL) and reduces the generation of VLDL.
https://www.chemwhat.com/gemfibrozil-cas-25812-30-0/
Comments
Post a Comment