IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
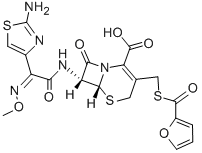
Product NameCeftiofurIUPAC Name(6R,7R)-7-amino]-3-(furan-2-carbonylsulfanylmethyl)-8-oxo-5-thia-1-azabicyclooct-2-ene-2-carboxylic acidMolecular StructureCAS Registry Number 80370-57-6EINECS Number1308068-626-2MDL NumberMFCD00864953SynonymsCeftiofur80370-57-6CeftiofurumNaxcelExcenelUNII-83JL932I1CCeftiofur crystalline free acidCeftiofurum HSDB 744583JL932I1CDTXCID5026702DTXSID7046702Ceftiofur (INN)Ceftiofurum (Latin)(6R,7R)-7-(2-(2-Amino-4-thiazolyl)glyoxylamido)-3-(mercaptomethyl)-8-oxo-5-thia-1-azabicyclo(4.2.0)oct-2-ene-2-carboxylic acid, 7(sup 2)-(Z)-(O-methyloxime), 2-furoate (ester)CEFTIOFUR CEFTIOFUR (MART.)CEFTIOFUR Naxcel (veterinary)Molecular FormulaC19H17N5O7S3Molecular Weight523.6InChIInChI=1S/C19H17N5O7S3/c1-30-23-11(9-7-34-19(20)21-9)14(25)22-12-15(26)24-13(17(27)28)8(5-32-16(12)24)6-33-18(29)10-3-2-4-31-10/h2-4,7,12,16H,5-6H2,1H3,(H2,20,21)(H,22,25)(H,27,28)/b23-11-/t12-,16-/m1/s1InChI KeyZBHXIWJRIFEVQY-IHMPYVIRSA-NIsomeric SMILESCO/N=C(/C1=CSC(=N1)N)C(=O)N23N(C2=O)C(=C(CS3)CSC(=O)C4=CC=CO4)C(=O)O
Patent InformationPatent IDTitlePublication DateWO2008/47376AN IMPROVED PROCESS FOR THE PREPARATION OF CEPHALOSPORIN ANTIBIOTIC2008
Physical Data
AppearanceOff-white or light yellowish powder
Description (Association (MCS))Solvent (Association (MCS))Temperature (Association (MCS)), °CPartner (Association (MCS))Adsorptionaq. phosphate buffer37pyrographite
Spectra
Description (IR Spectroscopy)Bands, Spectrum
Description (UV/VIS Spectroscopy)Spectrum
Route of Synthesis (ROS)
Route of Synthesis (ROS) of Ceftiofur CAS# 80370-57-6
ConditionsYieldStage #1: ceftiofur With triethylamine In tetrahydrofuran; water at -5 - 0℃; for 2h;Stage #2: With sodium 2-ethylhexanoic acid In tetrahydrofuran; water at 8 - 10℃; for 1h;Experimental Procedure4; 5 EXAMPLE-4 Preparation of ceftiofur sodium (Ib) from 7- 3-(mercaptomethyl)-8-oxo-5-thia-1-azabicyclo-oct-2-ene-2-carboxylic acid (ceftiofur) (Ia)140 ml of tetrahydrofuran (THF) and 4.0 ml of demineralised water (DMW) and 4.0 g (0.00764 moles) of 7- 3-(mercaptomethyl)-8-oxo-5-thia-1-azabicyclo-oct-2-ene-2-carboxylic acid (ceftiofur, Ia) were taken in a flask and stirred. The reaction mixture was cooled and 0.81 g (0.0080 moles) of triethyl amine (TEA) was added. Activated carbon (0.60 g) and sodium dithionite (0.04 g) were added and the reaction mixture filtered through celite bed and washed with a mixture of THF and DMW. The filtrate was passed through 0.2μ filter paper and washed with THF (100 ml). A solution of sodium-2-ethyl hexanoate (1.80 g, 0.01084 moles) was prepared in THF and passed through 0.2μ filter paper. This filtrate was added gradually to the solution of the triethylamine salt of ceftiofur, obtained above and the precipitated solid was filtered under nitrogen atmosphere. The wet cake was washed with THF, followed by washing with ethyl acetate (2×44 ml) and acetone (2×29 ml) and dried under nitrogen atmosphere for 30-45 minutes with occasional raking. The semi dry cake was dried in vacuum oven at 700 mmHg/40° C. till moisture content was 2.0% to give 3.48 g (87%) of Ceftiofur sodium (Ib) having purity of 98.0% 1000 ml of THF and 70.0 ml of demineralised water (DMW) and 100.0 g (0.191 moles) of 7- 3-(mercaptomethyl)-8-oxo-5-thia-1-azabicyclo- oct-2-ene-2-carboxylic acid (ceftiofur, Ia) were taken in a flask and stirred for 5 minutes. The mixture was cooled to -5° C. and 20.47 g (0.202 moles) of triethyl amine (TEA) was added at -5 to -3° C. in 60 minutes. Activated carbon (15 g) and sodium dithionite (1.0 g) were added and the reaction mixture was stirred at -5 to 0° C. for 60 minutes. The mixture was filtered through celite bed and washed with a mixture of THF and DMW (5:0.3 v/v; 530 mL). The filtrate was passed through 0.2μ filter paper and washed with THF (100 ml). A solution of sodium-2-ethyl hexanoate (50.78 g, 0.305 moles) was prepared in THF and passed through 0.2μ filter paper. This filtrate was added gradually to the solution of triethylamine salt of ceftiofur, obtained above and the mixture was stirred at at 8-10° C. for 60 minutes and the precipitated solid filtered under nitrogen atmosphere. The wet cake was slurry washed with THF (500 mL), followed by ethyl acetate (2×500 ml) and acetone (3×500 ml). The wet cake was dried under nitrogen atmosphere for 30-45 minutes with occasional raking. The semi dry cake was dried in vacuum oven at 700 mmHg/40° C. to give 88 g (0.88% w/w) of ceftiofur sodium (Ib), having a purity of 98%.87%With sodium 2-ethylhexanoic acid In tetrahydrofuran at 20℃; for 0.5h;Experimental Procedure Dichloromethane (20 ml) was charged and cooled to 0-5° C., 7--3--3-cephem-4-carboxylic acid p-methoxy benzyl ester (6.5 g) was added followed by trifluoroacetic acid (20 ml) at 0-5° C. The reaction mixture was stirred for 30-40 min. Anisole (12 ml) was also added at 5° C. and reaction was stirred at 5° C. for 1 hour, the reaction was checked by HPLC and after completion of reaction, water (100 ml) was added with stirring. The product was filtered and washed with water (100 ml) followed by dichloromethane (100 ml). The filtered solid was taken in tetrahydrofuran (100 ml) and dried over MgSO4. To the dried THF layer was added a solution of 5 g of 2-ethyl sodium hexanoate in THF (20 ml). The precipitated ceftiofur sodium was stirred for 30 minutes at 20° C. filtered and washed with acetone (100 ml). Yield = 5.0 g Purity = 99%
Safety and Hazards
GHS Hazard StatementsNot Classified
Other Data
HS CodeStorageUnder the room temperature and away from lightShelf Life2 yearsMarket Price
DruglikenessLipinski rules componentMolecular Weight523.571logP-2.103HBA9HBD3Matching Lipinski Rules3Veber rules componentPolar Surface Area (PSA)256.26Rotatable Bond (RotB)10Matching Veber Rules1
Use PatternCeftiofur a third-generation cephalosporin antibiotic designed for veterinary use. It has a broad spectrum of antibacterial activity, showing strong efficacy against Gram-positive and Gram-negative bacteria, as well as anaerobic bacteria. Its activity against Gram-positive bacteria is similar to or weaker than that of first-generation cephalosporins. However, it exhibits potent antibacterial activity against Gram-negative bacteria such as Escherichia coli, Salmonella typhi, Klebsiella pneumoniae, Enterobacter cloacae, and Streptococcus.
https://www.chemwhat.com/ceftiofur-cas-80370-57-6/
Comments
Post a Comment