IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
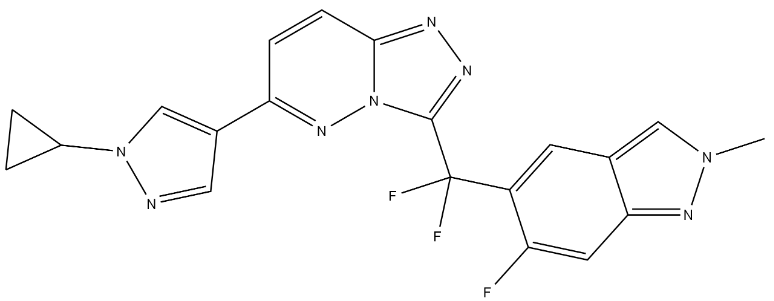
Product NamePLB-1001IUPAC Name6-(1-cyclopropylpyrazol-4-yl)-3--triazolopyridazineMolecular StructureCAS Registry Number 1440964-89-5SynonymsBozitinib1440964-89-5VebreltinibPLB-1001APL-101Bozitinib (PLB-1001)Vebreltinib 6-(1-cyclopropylpyrazol-4-yl)-3--triazolopyridazineCBT-101CBI-31032WZP8A9VFN1,2,4-Triazolo(4,3-b)pyridazine, 6-(1-cyclopropyl-1H-pyrazol-4-yl)-3-(difluoro(6- fluoro-2-methyl-2H-indazol-5-yl)methyl)-5-{triazolopyridazin-3-yl]difluoromethyl}-6-fluoro-2-methyl-2H-indazole6-(1-Cyclopropyl-1H-pyrazol-4-yl)-3-(difluoro(6-fluoro-2-methyl-2H-indazol-5- yl)methyl)-1,2,4-triazolo(4,3-b)pyridazine6-(1-Cyclopropyl-1H-pyrazol-4-yl)-3-(difluoro(6-fluoro-2-methyl-2H-indazol-5-yl)methyl)-triazolopyridazineUNII-2WZP8A9VFNVEBRELTINIB VEBRELTINIB CHEMBL4650443SCHEMBL15594471GTPL11677PLB1001QHXLXUIZUCJRKV-UHFFFAOYSA-NBDBM107096EX-A5644s6762WHO 11677AKOS040732634MS-27454HY-125017CS-0088607G16990US9695175, 44A937089PLB-1001;T-101; APL-101; CBI-3103Molecular FormulaC20H15F3N8Molecular Weight424.4InChIInChI=1S/C20H15F3N8/c1-29-9-11-6-14(15(21)7-17(11)27-29)20(22,23)19-26-25-18-5-4-16(28-31(18)19)12-8-24-30(10-12)13-2-3-13/h4-10,13H,2-3H2,1H3InChI KeyQHXLXUIZUCJRKV-UHFFFAOYSA-NIsomeric SMILESCN1C=C2C=C(C(=CC2=N1)F)C(C3=NN=C4N3N=C(C=C4)C5=CN(N=C5)C6CC6)(F)F
Patent InformationPatent IDTitlePublication DateWO2022/226168DIAGNOSTIC AND TREATMENT OF CANCER USING C-MET INHIBITOR2022WO2021/222045NOVEL PHARMACEUTICAL FORMULATION FOR C-MET INHIBITOR2021WO2019/161320CANCER TREATMENT USING COMBINATION OF NEUTROPHIL MODULATOR WITH MODULATOR OF IMMUNE CHECKPOINT2019WO2014/32498HIGHLY SELECTIVE C-MET INHIBITORS AS ANTICANCER AGENTS2014
Physical Data
Melting Point, °C 203221.16
Spectra
Route of Synthesis (ROS)
ConditionsYieldWith methanesulfonic acid; 1-methoxy-2-propanol In methanol at 90℃; for 16h;Experimental Procedure44 Example 44 : 6-(l-Cyclopropyl-lH-pyrazol-4-yl)-3-(difluoro(6-fluoro-2-inethyl-(2H- indazol-5-yl)methyl)-triazolopyridazineGeneral procedure: Example 44 : 6-(l-Cyclopropyl-lH-pyrazol-4-yl)-3-(difluoro(6-fluoro-2-inethyl-(2H- indazol-5-yl)methyl)-triazolopyridazine To a 100 n L round bottom flask is added l-methoxy-2-propanol (15 n L), methyl sulfonic acid (0.898 g, 9.4 mmol), 2,2-difluoro-2-(6-fluoro-2-methyl-2H-indazol-5-yl) acetohydrazide (2.01 g, 7.8 mmol), and 3-chloro-6-(l-cyclopropyl-lH-pyrazol-4-yl) pyridazine (1.81 g, 8.2 mmol). The reaction mixture is stirred at 90 °C for 16 hr. After removal of solvent, the residue is purified on a silica gel flash chromatography with methanol/dichloromethane (1 :20) to yield a light yellow solid (1.32 g, 40% yield). (MS: 425)40%
Safety and Hazards
GHS Hazard Statements
Other Data
HS CodeStorageStore at -20°C and away from lightShelf Life2 yearsMarket Price
DruglikenessLipinski rules componentMolecular Weight424.388logP5.934HBA8HBD0Matching Lipinski Rules3Veber rules componentPolar Surface Area (PSA)78.72Rotatable Bond (RotB)4Matching Veber Rules2
Use PatternPLB-1001 CAS#: 1440964-89-5 is a receptor tyrosine kinase inhibitor targeting the cell-mesenchymal-epithelial transition factor (c-MET), capable of inhibiting the proliferation of tumor cells with elevated c-MET expression. This drug can penetrate the blood-brain barrier and has been registered for 8 clinical trials domestically, with indications including non-small cell lung cancer (NSCLC), gliomas, and more.
https://www.chemwhat.com/plb-1001-cas-1440964-89-5/
Comments
Post a Comment