IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
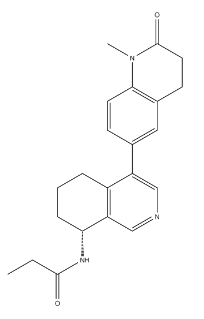
Product NameBaxdrostat (CIN-107)IUPAC NameN-propanamideMolecular StructureCAS Registry Number 1428652-17-8SynonymsBaxdrostatBaxdrostat NF3P9Z8J5Y1428652-17-8UNII-NF3P9Z8J5Y(+)-(R)-N-(4-(1-Methyl-2-oxo-1,2,3,4-tetrahydroquinolin-6-yl)-5,6,7,8-tetrahydroisoquinolin-8-yl)propionamideN-((8R)-5,6,7,8-Tetrahydro-4-(1,2,3,4-tetrahydro-1-methyl-2-oxo-6-quinolinyl)-8-isoquinolinyl)propanamidePropanamide, N-((8R)-5,6,7,8-tetrahydro-4-(1,2,3,4-tetrahydro-1-methyl-2-oxo-6-quinolinyl)-8-isoquinolinyl)-CIN107CHEMBL4113975SCHEMBL14799753GTPL12362CIN-107BDBM233806GLXC-26998MS-25786HY-132809RO6836191CS-0204056G18370US9353081, 3-1N-propanamideMolecular Formula C22H25N3O2Molecular Weight363.5InChIInChI=1S/C22H25N3O2/c1-3-21(26)24-19-6-4-5-16-17(12-23-13-18(16)19)14-7-9-20-15(11-14)8-10-22(27)25(20)2/h7,9,11-13,19H,3-6,8,10H2,1-2H3,(H,24,26)/t19-/m1/s1InChI KeyVDEUDSRUMNAXJG-LJQANCHMSA-NIsomeric SMILESCCC(=O)N1CCCC2=C1C=NC=C2C3=CC4=C(C=C3)N(C(=O)CC4)C
Patent InformationPatent IDTitlePublication DateUS2013/79365NEW BICYCLIC DIHYDROQUINOLINE-2-ONE DERIVATIVES2013WO2013/41591NEW BICYCLIC DIHYDROQUINOLINE-2-ONE DERIVATIVES2013
Physical Data
AppearanceSolid
Spectra
No data available
Route of Synthesis (ROS)
Route of Synthesis (ROS) of Baxdrostat (CIN-107) CAS 1428652-17-8
ConditionsYieldWith Chiralpak AD In n-heptane; isopropyl alcohol Resolution of racemate;Experimental Procedure2; 3 (-)-(R or S)-N-(4-(1-Methyl-2-oxo-1,2,3,4-tetrahydroquinolin-6-yl)-5,6,7,8-tetrahydroisoquinolin-8-yl)propionamide and (+)-(S or R)-N-(4-(1-Methyl-2-oxo-1,2,3,4-tetrahydroquinolin-6-yl)-5,6,7,8-tetrahydroisoquinolin-8-yl)propionamide(-)-(R or S)-N-(4-(1-Methyl-2-oxo-1,2,3,4-tetrahydroquinolin-6-yl)-5,6,7,8-tetrahydroisoquinolin-8-yl)propionamide and (+)-(S or R)-N-(4-(1-Methyl-2-oxo-1,2,3,4-tetrahydroquinolin-6-yl)-5,6,7,8-tetrahydroisoquinolin-8-yl)propionamideThe title compounds were prepared by chiral separation of (rac)-N-(4-(1-methyl-2-oxo-1,2,3,4-tetrahydroquinolin-6-yl)-5,6,7,8-tetrahydroisoquinolin-8-yl)propionamide (example 1) on a Chiralpak AD (40% 2-propanol in n-heptane) to give after precipitation from CH2Cl2 with n-pentane 28% of (-)-(R or S)-N-(4-(1-methyl-2-oxo-1,2,3,4-tetrahydroquinolin-6-yl)-5,6,7,8-tetrahydroisoquinolin-8-yl)propionamide (example 2) as off-white powder, MS: 364.2 (M+H+) and 26% of (+)-(S or R)-N-(4-(1-methyl-2-oxo-1,2,3,4-tetrahydroquinolin-6-yl)-5,6,7,8-tetrahydroisoquinolin-8-yl)propionamide (example 3) as off-white powder. MS: 364.2 (M+H+).A 28%B 26%
Safety and Hazards
Pictogram(s)SignalWarningGHS Hazard StatementsH302 (100%): Harmful if swallowed H361d (100%): Suspected of damaging the unborn child H373 (100%): May causes damage to organs through prolonged or repeated exposure Precautionary Statement CodesP203, P260, P264, P270, P280, P301+P317, P318, P319, P330, P405, and P501(The corresponding statement to each P-code can be found at the GHS Classification page.)
Other Data
HS CodeStorageUnder room temperature away from lightShelf Life2 yearsMarket Price
DruglikenessLipinski rules componentMolecular Weight363.459logP2.309HBA5HBD1Matching Lipinski Rules4Veber rules componentPolar Surface Area (PSA)62.3Rotatable Bond (RotB)4Matching Veber Rules2
Use PatternBaxdrostat (CIN-107) is an aldosterone synthase inhibitor (ASI) intended for antihypertensive treatment in refractory hypertension. It directly inhibits the synthesis of aldosterone, contributing to its blood pressure-lowering effects.
https://www.chemwhat.com/baxdrostat-cin-107-cas-1428652-17-8/
Comments
Post a Comment