IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
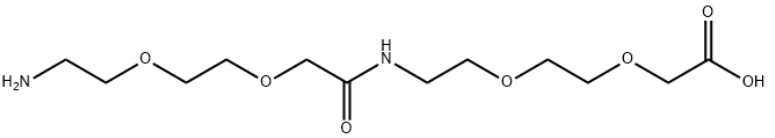
Product Name17-Amino-10-oxo-3,6,12,15-tetraoxa-9-azaheptadecanoic AcidIUPAC Name2-acetyl]amino]ethoxy]ethoxy]acetic acid Molecular StructureCAS Registry Number 1143516-05-5MDL NumberMFCD13184942SynonymsAeea-aeea1143516-05-517-amino-10-oxo-3,6,12,15-tetraoxa-9-azaheptadecan-1-oic acid2-acetyl]amino]ethoxy]ethoxy]acetic acid8-Amino-3,6-dioxaoctanoic acid dimerMFCD1318494217-Amino-10-oxo-3,6,12,15-tetraoxa-9-azaheptadecanoic acidH-Adoa-Adoa-OHSCHEMBL1257485AMY3342YQZVQKYXWPIKIX-UHFFFAOYSA-NDTXSID301191445EX-A5417H2N-PEG2-NH-PEG2-CH2COOHZB0899AKOS030213455HY-W125504AC-32527SY251169WS-03066CS-0183820P5002017-amino-10-oxo-3,6,12,15-tetraoxa-9-azaheptadecan-1-oicacidacetyl- amino}ethoxy)ethoxy]acetic acidacetyl-amino}ethoxy)ethoxy]acetic acidacetylamino}ethoxy)ethoxy]acetic acidacetylamino}ethoxy)ethoxy]acetic acid2-acetamido}ethoxy)ethoxy]acetic acid-acetylamino}-ethoxy)-ethoxy]-acetic acidMolecular FormulaC12H24N2O7Molecular Weight308.33InChIInChI=1S/C12H24N2O7/c13-1-3-18-5-7-20-9-11(15)14-2-4-19-6-8-21-10-12(16)17/h1-10,13H2,(H,14,15)(H,16,17)InChI KeyYQZVQKYXWPIKIX-UHFFFAOYSA-N Isomeric SMILESC(COCCOCC(=O)NCCOCCOCC(=O)O)N
Physical Data
AppearancePowder
Spectra
No data available
Route of Synthesis (ROS)
Route of Synthesis (ROS) of17-Amino-10-oxo-361215-tetraoxa-9-azaheptadecanoic Acid CAS 1143516-05-5
ConditionsYieldWith N-ethyl-N,N-diisopropylamine In ethanol at 20℃;Experimental ProcedureTo a solution of 2-(1 9-tert-l3utoxycarbonylnonade- canoylamino)pentanedioic acid 1 -tert-butyl ester 5-(2,5-di- oxopyrrolidin-1-yl) ester (2.50 g) and acetylamino}ethoxy)ethoxy]acetic acid (alternative name: H-OEG-OEG-OH)(1 .47 g) in ethanol (40 mE) was added DIPEA (1.26 mE). The mixture was stirred at room temperature overnight and then concentrated in vacuo. To the residue was added aqueous 0.1 N HC1 (150 mE) and ethyl acetate (200 mE). The layers were separated and the aqueous layer was extracted with ethyl acetate (100 mE). The combined organic layers were washed with water and brine, dried (magnesium sulphate) and concentrated in vacuo to give an oil, which crystallised on standing. Yield 96% (3.1 g). ECMS: Theoretical mass: 874.2.Found: 874.49.96%With N-ethyl-N,N-diisopropylamine In ethanol at 20℃;Experimental Procedure16.1 Step 1: 19-{(S)-1-tert-Butoxycarbonyl-3--methoxy}-ethoxy)-ethylcarbamoyl]-propylcarbamoyl}-nonadecanoic acid tert-butyl esterStep 1: 19-{(S)-1-tert-Butoxycarbonyl-3--methoxy}-ethoxy)-ethylcarbamoyl]-propylcarbamoyl}-nonadecanoic acid tert-butyl ester (0556) (0557) To a solution of 2-(19-tert-Butoxycarbonylnonadecanoylamino)pentanedioic acid 1-tert-butyl ester 5-(2,5-dioxopyrrolidin-1-yl) ester (2.50 g, (prepared similarly as described in WO 2005/012347) and acetylamino}ethoxy)ethoxy]acetic acid (1.47 g, alternative name: ∈-amino-3,6-dioxaoctanoic acid dimer, IRIS Biotech GmbH, Cat. No. PEG1221) in ethanol (40 ml) was added DIPEA (1.26 ml). The mixture was stirred at room temperature over night and then concentrated in vacuo. To the residue was added aqueous 0.1 N HCl (150 ml) and ethyl acetate (200 ml). The layers were separated and the aqueous layer was extracted with ethyl acetate (100 ml). The combined organic layers were washed with water and brine, dried (magnesium sulphate) and concentrated in vacuo to give an oil, which crystallized on standing. Yield 96% (3.1 g). LC-MS (electrospray): m/z=874.49.96%With N-ethyl-N,N-diisopropylamine In ethanol at 20℃;96%
Safety and Hazards
No data available
Other Data
TransportationStorage at 2~8°, Away from light.StorageStorage at 2~8°, Away from light.Shelf Life1 year
DruglikenessLipinski rules componentMolecular Weight308.332logP-2.772HBA9HBD3Matching Lipinski Rules4Veber rules componentPolar Surface Area (PSA)129.34Rotatable Bond (RotB)16Matching Veber Rules1
Use Pattern17-Amino-10-oxo-3,6,12,15-tetraoxa-9-azaheptadecanoic Acid CAS#: 1143516-05-5 as an intermediate in the synthesis of semaglutide, developing an efficient synthesis route with this intermediate could contribute to the cost-effectiveness of producing semaglutide.
https://www.chemwhat.com/aeea-aeea-cas-1143516-05-5/
Comments
Post a Comment