IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
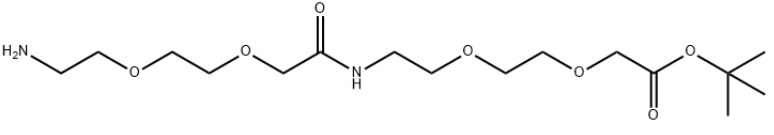
Product NameAEEA-AEEA-tBu/3,6,12,15-Tetraoxa-9-azaheptadecanoic acid,17-amino-10-oxo-,1,1-dimethylethyl esterMolecular StructureCAS Registry Number 2409545-30-6Molecular FormulaC16H32N2O7Molecular Weight364.43
Patent InformationPatent IDTitlePublication DateEP34986942019WO2019/1267302019US2018/2301572018
Physical Data
AppearancePowder
Spectra
Route of Synthesis (ROS)
Route of Synthesis (ROS) of 3 6 12 15-Tetraoxa-9-azaheptadecanoic acid 17-amino-10-oxo-1 1-dimethylethyl ester CAS 2409545-30-6
ConditionsYieldWith benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; diisopropylamine In dichloromethane at 0 - 20℃; for 6h;Experimental Procedure2.2-1.3 Step 3: Preparation of Compound II-1eCompound II-1d (305 mg, 830 umol)II-1a 8- (Fluorenylmethoxycarbonyl-amino) -3,6-dioxaotanoic acid (478 mg, 1.24 mmol)Dichloromethane (20 mL)After melting in, At 0 to room temperatureI- (3-dimethylaminopropyl) -3-ethylcarbodiimide hydrochloride (318 mg, 1.66 mmol), hydroxybenzotriazole (224 mg, 1.66 mmol) and diisopropylamine (423 μl, 2.49 mmol) Was added. The reaction mixture was stirred at room temperature for 6 hours. After completion of the reaction and extracted with distilled water and brine, the organic layer was dried over anhydrous sodium sulfate and concentrated under reduced pressure. The concentrated residue was purified by silica gel column chromatography (dichloromethane: methanol = 10: 1 volume ratio) to give the title compound II-1e (570 mg, 92%) as a yellow oil.92%
Safety and Hazards
No data availabile
Other Data
StorageStore at 2~8° for long time, away from lightShelf Life1 year
DruglikenessLipinski rules componentMolecular Weight364.439logP-1.151HBA9HBD2Matching Lipinski Rules4Veber rules componentPolar Surface Area (PSA)118.34Rotatable Bond (RotB)18Matching Veber Rules1
Use PatternAEEA-AEEA-tBu/3,6,12,15-Tetraoxa-9-azaheptadecanoic acid,17-amino-10-oxo-,1,1-dimethylethyl ester CAS#: 2409545-30-6 as an intermediate in the synthesis of semaglutide. And 3,6,12,15-Tetraoxa-9-azaheptadecanoic acid,17-amino-10-oxo-,1,1-dimethylethyl ester likely plays a crucial role in the production process. Semaglutide is a glucagon-like peptide-1 (GLP-1) receptor agonist used in the treatment of type 2 diabetes.
https://www.chemwhat.com/aeea-aeea-tbu-361215-tetraoxa-9-azaheptadecanoic-acid17-amino-10-oxo-11-dimethylethyl-ester-cas-2409545-30-6/
Comments
Post a Comment