IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
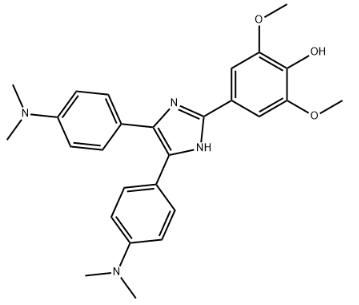
Product Name4,5-bis(4-dimethylaminophenyl)-2-(3,5-dimethoxy-4-hydroxyphenyl)imidazoleIUPAC Name4--1H-imidazol-2-yl]-2,6-dimethoxyphenol Molecular StructureCAS Registry Number 1886-13-1SynonymsPhotosensitizer-11886-13-14,5-bis(4-dimethylaminophenyl)-2-(3,5-dimethoxy-4-hydroxyphenyl)imidazole4--1H-imidazol-2-yl]-2,6-dimethoxyphenolphenol, 4--1H-imidazol-2-yl]-2,6-dimethoxy-4-{4,5-bis-1H-imidazol-2-yl}-2,6-dimethoxyphenolSCHEMBL175592SZXKSDXHODZTFS-UHFFFAOYSA-NAMY41390EX-A4874HY-D1293CS-0163526Molecular FormulaC27H30N4O3Molecular Weight458.6InChIInChI=1S/C27H30N4O3/c1-30(2)20-11-7-17(8-12-20)24-25(18-9-13-21(14-10-18)31(3)4)29-27(28-24)19-15-22(33-5)26(32)23(16-19)34-6/h7-16,32H,1-6H3,(H,28,29)InChI KeySZXKSDXHODZTFS-UHFFFAOYSA-N Isomeric SMILESCN(C)C1=CC=C(C=C1)C2=C(N=C(N2)C3=CC(=C(C(=C3)OC)O)OC)C4=CC=C(C=C4)N(C)C
Patent InformationPatent IDTitlePublication DateEP699905Leuco dye coating compositions1996US4166763Analysis of lactic acid or lactate using lactate oxidase1979
Physical Data
AppearanceWhite to off-white powder
Melting Point, °C 262 - 263
Spectra
Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Frequency (NMR Spectroscopy), MHzChemical shifts, Spectrum1Hdimethylsulfoxide-d6400Chemical shiftsSpectrum13Cdimethylsulfoxide-d6100.6
Description (UV/VIS Spectroscopy)Solvent (UV/VIS Spectroscopy)Absorption Maxima (UV/VIS), nmBand assignment, Spectrum1,4-dioxane315Band assignment, Spectrumtetrahydrofuran317Band assignment, Spectrumdichloromethane318Band assignment, Spectrumdimethyl sulfoxide318Band assignment, Spectrummethanol320
Route of Synthesis (ROS)
Route of Synthesis (ROS) of 4,5-bis(4-dimethylaminophenyl)-2-(3,5-dimethoxy-4-hydroxyphenyl)imidazole CAS 1886-13-1
ConditionsYieldWith ammonium acetate; glacial acetic acid In ethyl acetate for 6h; Reflux;Experimental ProcedureSyringaldehyde (3.07 g, 16.87 mmol), 1,2-Bis(4-Dimethylaminophenyl)-1,2-Ethanedione (5.00 g, 16.87 mmol) and NH4OAc (6.50 g,84.35 mmol) were dissolved in 150 mL glacial acetic acid, followed byrefluxing for 6 h. The reaction mixture was poured to ice-water andammonia was added to neutralize the mixture. 500 mL ethyl acetate wasadded to extract the crude product. The organic phase was dried withanhydrous MgSO4, and the solvent was removed under reduced pressure.The resulting crude product was purified via column chromatography(silica gel, pure ethyl acetate), given the pure product as a whitesolid: yield (4.5 g, 58%). 1H NMR (400 MHz, DMSO-d6): δ 12.10 (s, 1H,NH), 8.53 (s, 1H, OH), 7.28-7.41 (m, 6H, ArH), 6.70-6.72 (m, 4H, ArH),3.84 (s, 6H, OCH3), 2.91 (s, 12H, N(CH3) 2) ppm. 13C NMR (100.6 MHz,DMSO-d6): δ 148.6, 145.2, 136.1, 129.5, 128.1, 124.4, 121.8, 112.5,103.1, 56.5, 19.0. HRMS-EI m/z: calcd for C27H30N4O3 + H+: 459.2396;measured: 459.2374. CCDC: 2130100.58%
Safety and Hazards
No data available
Other Data
TransportationStore at -20°C for long time, in container tightly sealed; Protect from light.HS CodeStorageStore at -20°C for long time, in container tightly sealed; Protect from light.Shelf Life1 yearMarket Price
DruglikenessLipinski rules componentMolecular Weight458.56logP5.706HBA4HBD2Matching Lipinski Rules3Veber rules componentPolar Surface Area (PSA)73.85Rotatable Bond (RotB)7Matching Veber Rules2
Quantitative Results1 of 2Comment (Pharmacological Data)Bioactivities presentReferenceLeuco dye coating compositions2 of 2Comment (Pharmacological Data)Bioactivities presentReferenceUsing the analysis element and dry immunoassy assay method
Use Pattern4,5-bis(4-dimethylaminophenyl)-2-(3,5-dimethoxy-4-hydroxyphenyl)imidazole CAS:# 1886-13-1, due to the presence of the imidazole ring and aromatic rings, the compound may exhibit photosensitive properties, undergoing chemical reactions upon exposure to light. This property suggests potential applications in photosensitive materials or photonic devices.
https://www.chemwhat.com/45-bis4-dimethylaminophenyl-2-35-dimethoxy-4-hydroxyphenylimidazole-cas-1886-13-1/
Comments
Post a Comment