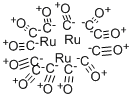
IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
Product NameRUTHENIUM CARBONYLMolecular StructureCAS Registry Number 15243-33-1MDL NumberMFCD00011209Molecular FormulaC5H6N2Molecular Weight94.116
Physical Data
SolubilityIt is soluble in water as well as soluble in alcohol, benzene.
Melting Point, °C 144 - 145154 - 155154 - 156
Density, g·cm-3Measurement Temperature, °C2.56-173.162.552-173.162.552-153.162.486
Spectra
Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Temperature (NMR Spectroscopy), °C Frequency (NMR Spectroscopy), MHzSpectrum13CChemical shifts, Spectrum13Cbenzene-d6100.6Spectrum, Linewidth of NMR absorptionotherneat (no solvent, solid phase)othertetrahydrofuran39.84Spectrum13Cnot given
Description (IR Spectroscopy)Solvent (IR Spectroscopy)Intensity of IR bands, Bands, Spectrumpotassium bromideBandsn-heptaneBandshexane
Description (UV/VIS Spectroscopy)Solvent (UV/VIS Spectroscopy)Reflection spectrum, SpectrumSpectrumBand assignment, Spectrumethyl acetateSpectrumdichloromethane
Route of Synthesis (ROS)
Route of Synthesis (ROS) of Ruthenium carbonyl CAS 15243-33-1
ConditionsYieldWith triethylamine In propan-1-ol; water at 85℃; under 2625.26 Torr; Concentration; Reagent/catalyst; Solvent; Temperature; Pressure; Autoclave;Experimental ProcedureGeneral procedure: 10031] In this embodiment, a synthesis test to confirm the presence or absence of the effect by the addition of an amine to the reaction system for DCR synthesis was performed. The production process of DCR is as follows. Ruthenium chloride (manufactured by Tanaka Kikinzoku Kogyo K. K., ruthenium chloride content: 38.67 wt %, chlorine content: 47.4 wt %) and 1 -propanol were mixed and stirred to prepare a ruthenium chloride solution, and this was introduced into an autoclave (made of steel) having a capacity of 100 ml of the reaction vessel. Thereafier, an amine was added into the reaction vessel and further carbon monoxide was air tightly introduced into the reaction vessel until to have a predetermined reaction pressure. Thereafter, the temperature was raised to the reaction temperature while maintaining the predetermined reaction pressure with carbon monoxide, and the synthesis reaction of DCR was allowed to proceed. The solution was stirred during the reaction. The reaction conditions in the present embodiment were as follows. In the present embodiment, DCR was synthesized using a plurality of amines which has different numbers of carbon atoms. In addition, the possibility of the synthesis of DCR in the case of not adding an aminewas also investigated; The relation between the water content of 1-pro-panol before being used for the preparation of the rutheniumchloride solution and the yield of DCR was investigated. Theproduction process of DCR other than this was the same as inthe first embodiment. The reaction conditions were as follows. The measurement results of the yield of DCR in this testare shown in Table 5.10090] Ruthenium chloride: 1.58 g (Ru: 0.61 g)10091] 1 -propanol: 60 mE (water content: dry, 0.5 wt %, 1.0wt%, 3.Owt%, 5.Owt%, lOwt%and3owt%)10092] Triethylamine: 1.3 equivalents (molar equivalentwith respect to chlorine)10093] Reaction pressure: 0.35 MPaj0094]j0095]j0096]Reaction temperature: 85° C.Reaction time: 10 to 60 hoursStirring speed: 300 rpm. As can be seen from Table 5, there is a possibility that the yield of DCR is affected by the water content in the solvent. Moreover, it is not possible to obtain a practical yield of DCR in the case of using a solvent which contains water at 10% or more. From this fact, it is preferable to set the water content in the solvent to 5% by mass or less. However, it is not intended to require a dry state (water content of approximately 0%), and it is possible to obtain a high yield using a solvent having a water content of 1% or less.92.5%In methanol at 125℃; under 48754.9 Torr; for 8h; Autoclave;
Safety and Hazards
No data available
Other Data
No data available
DruglikenessLipinski rules componentMolecular Weight639.335logPHBA12HBD0Matching Lipinski Rules1Veber rules componentPolar Surface Area (PSA)0Rotatable Bond (RotB)0Matching Veber Rules2
Use PatternRUTHENIUM CARBONYL is often used as a catalyst in organic synthesis. It facilitates the reaction between carbon monoxide and organic compounds, leading to the production of carbonyl compounds. These reactions are significant in the synthesis of pharmaceuticals and other organic compounds. And RUTHENIUM CARBONYL can also be employed as a catalyst in hydrogen transfer reactions, participating in reactions that enable the effective transfer of hydrogen in organic synthesis.
https://www.chemwhat.com/ruthenium-carbonyl-cas-15243-33-1/
Comments
Post a Comment