IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
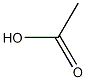
Product NameAcetic acidIUPAC Nameacetic acid Molecular StructureCAS Registry Number 64-19-7EINECS Number200-580-7MDL NumberMFCD00036152Beilstein Registry Number506007Synonymsacetic acidethanoic acid64-19-7Ethylic acidVinegar acidAcetic acid glacialGlacial acetic acidAcetic acid, glacialMethanecarboxylic acidAcetasolEssigsaeureAcide acetiqueVinegarPyroligneous acidAzijnzuurAceticum acidumAcido aceticoOctowy kwasAci-jelHOAcethoic acidKyselina octovaAcOHAzijnzuur Ethanoic acid monomerAceticEssigsaeure Caswell No. 003Otic TridesilonOctowy kwas Acetic acid (natural)Orthoacetic acidAcide acetique Acido acetico FEMA No. 2006Kyselina octova MeCOOHOtic DomeboroAcidum aceticum glacialeAcidum aceticumCH3-COOHacetic acid-CH3CO2HUN2789UN2790EPA Pesticide Chemical Code 044001NSC 132953NSC-132953NSC-406306BRN 0506007Acetic acid, dilutedAcetic acid-17O2INS NO.260Acetic acid DTXSID5024394MeCO2HCHEBI:15366AI3-02394CH3COOHINS-260Q40Q9N063PE-26010.Methanecarboxylic acidCHEMBL539NSC-111201NSC-112209NSC-115870NSC-127175Acetic acid-2-13C,d4INS No. 260DTXCID304394E 260Acetic-13C2 acid (8CI,9CI)EthanoatShotgunMFCD00036152Acetic acid, of a concentration of more than 10 per cent, by weight, of acetic acid68475-71-8Acetic Acid-2-13C-2,2,2-d3C2:0acetyl alcoholOrlexVosol63459-47-2ACETIC-1-13C-2-D3 ACID-1 H (D)WLN: QV1ACETIC ACID (MART.)ACETIC ACID Acetic acid, >=99.7%285977-76-6FEMA Number 2006ACETIC-13C2-2-D3 ACID, 97 ATOM % 13C, 97 ATOM % DAcetic acid, ACS reagent, >=99.7%57745-60-5ACYHSDB 40CCRIS 595279562-15-5methane carboxylic acidEINECS 200-580-7Acetic acid 0.25% in plastic containerEssigsaureEthylateacetic aicdacetic-acidGlacial acetateacetic cidactic acidUNII-Q40Q9N063Pacetic -acidDistilled vinegarMethanecarboxylateAcetic acid, glacial Acetasol (TN)Acetic acid,glacialVinegar (Salt/Mix)3,3'-(1,4-phenylene)dipropiolic acidHOOCCH3546-67-8Acetic acid LC/MS GradeACETIC ACID ACETIC ACID Acetic acid, ACS reagentbmse000191bmse000817bmse000857Otic Domeboro (Salt/Mix)EC 200-580-7Acetic acid (JP17/NF)ACETIC ACID } -->
Physical Data
AppearanceTransparent ilquid,no visible impuritiesMoisture≤0.115%(m/m)Acetaldehyde≤0.030%(m/m)Permanganate Time≥30min
Melting Point, °C 16.716.616.5316.616.5 - 16.5516.5
Boiling Point, °CPressure (Boiling Point), Torr117.94760.051117.79760.014117.9118.28760.051118117.9760.051
Density, g·cm-3Measurement Temperature, °C1.04919.991.0216144.991.0270439.991.0326134.991.0384429.991.043924.991.046924.99
Description (Association (MCS))Solvent (Association (MCS))Temperature (Association (MCS)), °CPartner (Association (MCS))Adsorption isotherm25CF3 functionalized MIL-53(Al)AdsorptionC30H28N4O4AdsorptionSAPO-34Adsorptionneat (no solvent, gas phase)aluminum oxide
Spectra
Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Temperature (NMR Spectroscopy), °C Frequency (NMR Spectroscopy), MHzChemical shifts, Spectrum13CChemical shifts, Spectrum1Hwater-d2Chemical shifts1Hheavy water400Chemical shifts1H-163.16500Chemical shifts1Hheavy water400.1
Description (IR Spectroscopy)Solvent (IR Spectroscopy)Temperature (IR Spectroscopy), °CSpectrum ATR (attenuated total reflectance), Bands, SpectrumSpectrumgas-195.16
Description (UV/VIS Spectroscopy)Solvent (UV/VIS Spectroscopy)Comment (UV/VIS Spectroscopy)SpectrumH2O175 - 350 nmSpectrum190 - 240 nmSpectrum180 - 250 nmAbsorption maxima
Route of Synthesis (ROS)
Route of Synthesis (ROS) of Acetic acid CAS 64-19-7
ConditionsYieldWith methanesulfonic acid In cyclohexane; butanone at 80℃; under 760.051 Torr; Reagent/catalyst; Temperature; Solvent; Large scale;Experimental ProcedureEsterification raw material 9.2kgGlycerin and water-carrying agent 3kg butanone were added to a 30L atmospheric pressure reaction kettle, and the esterification reaction was carried out.It is a reversible reaction and produces water. In order to increase the conversion rate, water can be used to separate water from the reaction system. The material that can be used as a water-carrying agent must react with water to produce an azeotrope so that the water is more easily distilled out, and the solubility in water is small, and in this scheme, cyclohexane is used as a water-carrying agent.In addition, methanesulfonic acid was added to the reaction vessel as a catalyst for the esterification reaction, and the reaction vessel was heated to 80 ° C as a reaction temperature, and then 6 kg of another esterification raw material acetic acid was gradually added to the reaction vessel to cause it to occur. The esterification reaction produces glycerol monoacetate and water. Moreover, the reaction can be simultaneously applied to a reflux system with a water separator, and the water phase of the lower layer is separated by a water separator to obtain an oil phase of the upper layer, wherein the target product glycerin monoacetate is contained in the oil phase.The product in the reaction system can also be subjected to purification treatment. Specifically, the obtained oil phase is separated by a rectification column, and the water-carrying cyclohexane and the unreacted esterified raw material acetic acid in the oil phase are removed by vacuum distillation, and the heavy fraction at the bottom of the bottom is obtained. Pure glycerol monoacetate, the corresponding yield of this product is as high as 89.2%.89.2%
Safety and Hazards
Pictogram(s)SignalDangerGHS Hazard StatementsH226 (99.72%): Flammable liquid and vapor H314 (99.96%): Causes severe skin burns and eye damage H318 (14.58%): Causes serious eye damage Precautionary Statement CodesP210, P233, P240, P241, P242, P243, P260, P264, P264+P265, P280, P301+P330+P331, P302+P361+P354, P303+P361+P353, P304+P340, P305+P354+P338, P316, P317, P321, P363, P370+P378, P403+P235, P405, and P501(The corresponding statement to each P-code can be found at the GHS Classification page.)
Other Data
No data available
DruglikenessLipinski rules componentMolecular Weight60.0526logP-0.08HBA2HBD1Matching Lipinski Rules4Veber rules componentPolar Surface Area (PSA)37.3Rotatable Bond (RotB)0Matching Veber Rules2
Quantitative Results1 of 4,093Comment (Pharmacological Data)Bioactivities presentReferenceDerivatives of 2-(iminomethyl)amino-phenyl, their preparation, their use as medicaments and the pharmaceutical compositions containing them2 of 4,093Comment (Pharmacological Data)Bioactivities presentReferenceProcess for purifying carbon dioxide-containing gas streams3 of 4,093Comment (Pharmacological Data)Bioactivities presentReferenceSubstituted hydrazine mitomycin analogs4 of 4,093Comment (Pharmacological Data)Bioactivities presentReferenceVitamin D3 metabolites. I. Synthesis of 25-hydroxycholesterol.5 of 4,093Comment (Pharmacological Data)Bioactivities presentReferenceCob(I)alamin as catalyst. IV. Reduction of α,β-unsaturated nitriles6 of 4,093Comment (Pharmacological Data)Bioactivities presentReferencePreparation of 29-acetoxy-30-norlupan-20-one derivatives with the substituted ring C7 of 4,093Comment (Pharmacological Data)Bioactivities presentReferenceSTRUCTURE OF HELICOBASIDIN, A NOVEL BENZOQUINONE FROM HELICOBASIDIUM8 of 4,093Comment (Pharmacological Data)Bioactivities presentReferenceBile acids and steroids. XXXV. Some A-ring aromatic steriods having oxygen functions at C-16 and their pharmacological activities.9 of 4,093Comment (Pharmacological Data)Bioactivities presentReferenceStudies on telomers and oligomers of vinylene carbonate. VI. Stereoselective conversion of vinylene carbonate telomers to trans unsaturated phosphate esters and their chemical behaviors10 of 10Comment (Pharmacological Data)Bioactivities presentReferenceSubstituted cyclopropyl benzamides and pharmaceutical preparations and methods of use employing such compounds
Use PatternACETIC ACID is manufacture of vinyl acetate, acetic anhydride, acetic ester, acetate, ethyl cellulose, and chloro acetic acid. It can also be used in the field of synthetic fiber, binding agent, pharmacy, fertilizer and dyeing raw material, and in the field of plastic, rubber and printing as solvent.
https://www.chemwhat.com/acetic-acid-cas-64-19-7/
Comments
Post a Comment