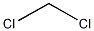
IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
Product NameDichloromethaneIUPAC Namedichloromethane Molecular StructureCAS Registry Number 75-09-2EINECS Number200-838-9MDL NumberMFCD00000881Beilstein Registry Number1730800SynonymsDICHLOROMETHANEMethylene chloride75-09-2Methylene dichlorideMethane, dichloro-Methylene bichlorideMethane dichlorideSolaesthinSolmethineNarkotilFreon 30Aerothene MMMetylenu chlorekChlorure de methyleneDichlormethanMetaclenSoleana VDAKhladon 30CH2Cl2F 30 (chlorocarbon)Methylenum chloratumR30 (refrigerant)RCRA waste number U080Caswell No. 568dichloro-methaneNCI-C50102R 30HCC 30Chloride, MethyleneBichloride, MethyleneDichloride, MethyleneHSDB 66CCRIS 392dichlormethaneMethokloneMethylenchloridSalesthinNSC 406122UN 1593dichloro methaneChlorodorm DF 30EINECS 200-838-9UNII-588X2YUY0AEPA Pesticide Chemical Code 042004BRN 1730800588X2YUY0ADTXSID0020868CHEBI:15767AI3-01773Methylene chloride NSC-406122Dichloromethane, HPLC GradeDTXCID40868EC 200-838-94-01-00-00035 (Beilstein Handbook Reference)MFCD00000881NSC406122Methylene chloride (NF)DICHLOROMETHANE (IARC)DICHLOROMETHANE DICHLOROMETHANE (MART.)DICHLOROMETHANE METHYLENE CHLORIDE (II)METHYLENE CHLORIDE Metylenu chlorek METHYLENE CHLORIDE (EP MONOGRAPH)METHYLENE CHLORIDE Methylene chloride; Dichloromethane; DCMMFCD00000882Chlorure de methylene Dichloromethane (Methylene Chloride)UN1593DICHLOROMETHANE, NFRCRA waste no. U080DICHLOROMETHANE, ACSdichioromethanedichlormetanedichloromeihanedichlorometandichlorometanedichloromethandichoromethanedicloromethanemethylenchoridemetylenchlorideAerotheneDriveritNevolindichlor-methanedichlorometlianedichlorornethanedicliloromethanemethylenchloridemethylenechloridmethYIenechloriddi-chloromethanedichloromethane-methlyenechloridemethylenechloridemethlene chloridemethyene chloridemethylen chloridemethylene chloriemethylene cloridemetylene chlorideMethylene choridemehtylene chloridemethlyene chloridemethylene,chloridemethylene-chloridedichloro -methanedichloro- methanemethylenedichlorideDistillex DS3Dichloromethane, ACS reagent, >=99.5%, contains 40-150 ppm amylene as stabilizerMolecular FormulaCH2Cl2Molecular Weight84.93InChIInChI=1S/CH2Cl2/c2-1-3/h1H2 InChI KeyYMWUJEATGCHHMB-UHFFFAOYSA-N Isomeric SMILESC(Cl)Cl
Patent InformationPatent IDTitlePublication DateCN113979916Synthesis method of polyhalogenated azaspirocyclohexadienone compound2022CN115368371Chiral triazine heterocyclic ring screwing 1, 4-benzodiazep-2-ketone compound and preparation method of chiral triazine heterocyclic ring screwing 1, 4-benzodiazep-2-ketone compound2022CN113429330Method for preparing 2-pyrrolidone derivative through three-component tandem cyclization reaction under copper catalysis2021CN110818673Synthetic method of cyclic methylene disulfonate2020CN111116363Preparation method of carboxylic ester compounds2019
Physical Data
AppearanceTransparent liquid,no visible impuritiesAcidity(as acetic acid),%(m/m) ≤0.0004Evaporated Residue,%(m/m)≤0.0005Chroma APHA (Pt-Co)10
Melting Point, °C -96.66-97-96.7217 - 219-95.15
Boiling Point, °CPressure (Boiling Point), Torr39.632585.23939.6760.0514040.1724.572
Density, g·cm-3Measurement Temperature, °C2.093-173.161.297234.991.331.316524.840.001321624.99
Description (Association (MCS))Temperature (Association (MCS)), °CAdsorption isotherm19.84Adsorption and desorption isotherms24.84Adsorption and desorption isotherms24.84Adsorption isotherm
Spectra
Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Temperature (NMR Spectroscopy), °C Chemical shifts, Spectrum1HacetonitrileSpectrum1Hdimethylsulfoxide-d6NMR with shift reagents, Spectrum1Hchloroform-d124.84NMR with shift reagents, Spectrum1Hchloroform-d134.84NMR with shift reagents, Spectrum 1Hchloroform-d144.84
Description (IR Spectroscopy)Solvent (IR Spectroscopy)Bands, SpectrumBands, SpectrumBandsneat (no solvent)ATR (attenuated total reflectance), Bands, Spectrum
Description (UV/VIS Spectroscopy)Solvent (UV/VIS Spectroscopy)SpectrumUV two-photon absorption, SpectrumSpectrumN,N-dimethyl-formamideSpectrumwaterSpectrumneat liquid
Route of Synthesis (ROS)
Route of Synthesis (ROS) of Dichloromethane CAS 75-09-2
ConditionsYieldWith sodium hydroxide In dimethyl sulfoxide at 50 -100℃; for 5.5h; Reagent/catalyst; Temperature; Microwave irradiation;Experimental Procedure110ml DMSO, 30 ml methylene chloride and 56g of sodium hydroxide were added into 500ml three-necked flask, heated by microwave heating to 50°C, 27.5g of catechol dissolved in 110 ml DMSO solution were added dropwise, the temperature control system was not exceeds 100° C for 5 hours. After dropwise addition, 20ml of dichloromethane was added dropwise, maintained at 90 °C for half an hour, cooled to room temperature, filtered to remove sodium chloride, 400ml water was added, dichloromethane and pepper ring were separated using oil separator, recovered water was started to distill, the distilled aqueous phase was extracted with appropriate amount of dichloromethane, the extracted aqueous phase was recycled at a distillation temperature of 100 deg.C, DMSO was recovered by distillation, the recovered DMSO can be recycled. About 10g remained. The oil-water separation was carried out to give the organic phase ring 36g, purity 97% gas, more than 95% yield.95%In 1-methyl-pyrrolidin-2-one Reagent/catalyst;Experimental ProcedureRepeat the preparation of the catalyst in Example 1, add 62.63 g of the compound alkali prepared above to the four-necked bottle, the composite base contains 59.63 g of inorganic base (potassium carbonate, magnesium oxide, the mass ratio of potassium silicate and barium chloride is 45: 1/2: 1/2: 2 × 10-6) and 3 g sodium methoxide composite catalyst, and 128.89 g NMP (1.300 mol) is added, during the dropwise addition of catechol solution, 44.23g (0.402mol) and 129.26g NMP (1.304mol) and 140.14g dichloromethane (1.650mol) were added. Add 64.53g (0.760mol) of methylene chloride, a total of 47.07g of benzodioxole (0.385mol) was synthesized by gas chromatography, and 0.37g of catechol remained unreacted. The calculated conversion rate of catechol is 99.16%, the selectivity of benzodioxole is 95.49%, and the synthesis yield is 94.69%.94.69%With sodium hydroxide In dimethyl sulfoxide at 90 -120℃; for 0.5h; Temperature; Solvent; Reagent/catalyst;Experimental Procedure440 ml of DMSO,Dichloromethane 120 ml, 230 g of sodium hydroxide was charged into a 2-liter three-necked flask, Heating up to 95 ° C, A solution of 110 g of catechol dissolved in 440 ml of DMSO was initially added dropwise to control the temperature of the system not to exceed 120 ° C, Dropping 3 to 4 hours, After dropping and dropping 90 ml of dichloromethane, Dripping at 110 ~ 115 reflux insulation for half an hour, Cooled to room temperature, Filtered to remove sodium chloride, Add water 400ml, The residual methylene chloride and pepper rings were separated using an oil-water separator, Separated from the basic no obvious oil phase distillation out, Began to recycle water,The distilled water phase was extracted with an appropriate amount of dichloromethane, The extracted aqueous phase can be recycled. Distilled to a top temperature of 100 ° C, Start switching the distillation to recover DMSO, Recovered DMSO can be recycled, Remnant about 90g. Oil and water separation to get organic phase pepper ring 129g, Gas purity of 97% Yield 85.4%.85.4%
Safety and Hazards
Pictogram(s)SignalWarningGHS Hazard StatementsH351: Suspected of causing cancer Precautionary Statement CodesP203, P280, P318, P405, and P501(The corresponding statement to each P-code can be found at the GHS Classification page.)
Other Data
No data available
DruglikenessLipinski rules componentMolecular Weight84.9329logP1.519HBA0HBD0Matching Lipinski Rules4Veber rules componentPolar Surface Area (PSA)0Rotatable Bond (RotB)0Matching Veber Rules2
Quantitative Results 1 of 1,199Comment (Pharmacological Data)Bioactivities presentReferenceMETHOD FOR STORING QUATERNARY AMMONIUM SALT2 of 1,199Comment (Pharmacological Data)Bioactivities presentReferenceCyclopentano (h) or (f) 1,2,3,4-tetrahydroisoquinolines3 of 1,199Comment (Pharmacological Data)Bioactivities presentReferenceAnalgesic N-be4 of 1,199Comment (Pharmacological Data)Bioactivities presentReferenceSulfur-substituted phenoxypyridines having antiviral activity5 of 1,199Comment (Pharmacological Data)Bioactivities presentReferencePROCESS FOR PREPARING 1,2,5-THIADIAZOLES6 of 1,199Comment (Pharmacological Data)Bioactivities presentReferencePROTEASE INHIBITORS
Use PatternMETHYLENE CHLORIDE used in a medicine and pestcide intermediates. And it is used to decaffeinate coffee and tea,as well as to prepare extracts of hops and other flavorings. And olvent in production of antibiotics, vitamin,film footage,aerosols. METHYLENE CHLORIDE used in the production of flame retardant series products.
https://www.chemwhat.com/dichloromethane-cas-75-09-2-2/
Comments
Post a Comment