IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
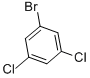
Product Name1-Bromo-3,5-dichlorobenzeneIUPAC Name1-bromo-3,5-dichlorobenzene Molecular StructureCAS Registry Number 19752-55-7EINECS Number243-270-7MDL NumberMFCD00000584Synonyms1-BROMO-3,5-DICHLOROBENZENE19752-55-73,5-DichlorobromobenzeneBenzene, 1-bromo-3,5-dichloro-1-bromo-3,5-dichloro-benzeneMFCD00000584benzene, 1-bromo-3,5-dichloroMaybridge1_0008813,5-Dichloro bromobenzene3,5-dichloro-1-bromobenzeneEINECS 243-270-7l-Brom-3,5-dichlorbenzol3,5-dichloro bromo benzeneSCHEMBL114694(3,5-Dichlorophenyl) bromideDTXSID1066524HMS544A01STR07058BTB 03107CCG-507891-Bromo-3,5-dichlorobenzene, 98%AKOS009156912AC-7762CS-W013037SY006090AM20060125B2223FT-0607474EN300-42477SR-01000640136-1W-107679BENZYL4,6-O-BENZYLIDENE-ALPHA-D-GALACTOPYRANOSIDEF0001-0702Z425865630InChI=1/C6H3BrCl2/c7-4-1-5(8)3-6(9)2-4/h1-3Molecular FormulaC6H3BrCl2Molecular Weight225.89InChIInChI=1S/C6H3BrCl2/c7-4-1-5(8)3-6(9)2-4/h1-3HInChI KeyDZHFFMWJXJBBRG-UHFFFAOYSA-N Isomeric SMILESC1=C(C=C(C=C1Cl)Br)Cl
Patent InformationPatent IDTitlePublication DateCN115850021Preparation method of polychlorinated biphenyl standard substance2023CN116425623Method for synthesizing 3,5-dichloro-4-methylbenzoic acid by one-pot method2023WO2022/61917SYNTHESIS METHOD FOR 3',5'-DICHLORO-2,2,2-TRIFLUOROACETOPHENONE2022CN112028752Synthesis method of 3', 5'-dichloro-2, 2, 2-trifluoroacetophenone2020
Physical Data
AppearanceWhite to light yellow crystalline powderMoisture ≤0.2%
Melting Point, °C Solvent (Melting Point) 76 - 76.5ethanol74ethanol77.582 - 84
Boiling Point, °CPressure (Boiling Point), Torr227756232757
Spectra
Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Temperature (NMR Spectroscopy), °C Frequency (NMR Spectroscopy), MHzChemical shifts, Spectrum1Hchloroform-d1400Chemical shifts, Spectrum13Cchloroform-d1100Chemical shifts1HCDCl3Chemical shifts35Cl-196.2
Description (IR Spectroscopy)Comment (IR Spectroscopy)Bands3120 - 428 cm**(-1)IR
Description (UV/VIS Spectroscopy)Solvent (UV/VIS Spectroscopy)Comment (UV/VIS Spectroscopy)Absorption Maxima (UV/VIS), nmExt./Abs. Coefficient, l·mol-1cm-1Absorption maximaH2O, H2SO4Ratio of solvents: 66percent2585740Absorption maximaH2O, NaOHRatio of solvents: 0.1N232, 2908600, 3120
Route of Synthesis (ROS)
Route of Synthesis (ROS) of 1-Bromo-3,5-dichlorobenzene CAS 19752-55-7
ConditionsYieldWith potassium carbonate; palladium diacetate; tris-(o-tolyl)phosphine In ethanol; water; toluene at 80℃; for 7h; Suzuki-Miyaura reaction; Inert atmosphere;99%With tetrakis(triphenylphosphine) palladium(0); potassium carbonate In tetrahydrofuran; water at 80℃;Experimental Procedure 9-Phenyl-9H-carbazol-3-yl)boronic acid (1 equiv), 1-bromo-3,5-dichlorobenzene (1 equiv), Pd(PPh3)4 (0.05 equiv) and K2CO3 ( 3 eq) was dissolved in a 2:1 mixed solution of water and THF, and stirred at 80°C for 12 hours.After cooling and washing three times with ethyl acetate and water, the organic layer was separated therefrom,and dried over MgSO4 and under reduced pressure.The obtained product was purified by column chromatography using dichloromethane (MC) and n-hexane,to obtain intermediate 2-1.(yield: 81%)80%
Safety and Hazards
Pictogram(s)SignalWarningGHS Hazard StatementsH315 (100%): Causes skin irritation H319 (100%): Causes serious eye irritation H335 (100%): May cause respiratory irritation Precautionary Statement CodesP261, P264, P264+P265, P271, P280, P302+P352, P304+P340, P305+P351+P338, P319, P321, P332+P317, P337+P317, P362+P364, P403+P233, P405, and P501(The corresponding statement to each P-code can be found at the GHS Classification page.)
Other Data
TransportationStore at room temperature for long time; sealed and keep away from light.StorageStore at room temperature for long time; sealed and keep away from light.Shelf Life2 years
DruglikenessLipinski rules componentMolecular Weight225.9logP4.064HBA0HBD0Matching Lipinski Rules4Veber rules componentPolar Surface Area (PSA)0Rotatable Bond (RotB)0Matching Veber Rules2
BioactivityIn vitro: EfficacyQuantitative Results
Toxicity/Safety PharmacologyQuantitative Results
Use Pattern1-Bromo-3,5-dichlorobenzene CAS#: 19752-55-7 can serve as an intermediate in organic synthesis. And it be used in chemical laboratories for research and synthetic reactions. It can be employed to prepare other organic compounds for studying their properties and reactions.
https://www.chemwhat.com/1-bromo-35-dichlorobenzene-cas-19752-55-7/
Comments
Post a Comment