IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
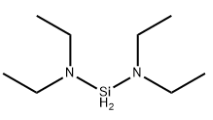
Product NameBis(diethylamino)dihydrosilaneMolecular StructureCAS Registry Number 27804-64-4MDL NumberMFCD19105220Synonymsbis(diethylamino)silane27804-64-4Silanediamine, N,N,N',N'-tetraethyl-MFCD19105220SCHEMBL35573DTXSID401301011N,N,N',N'-TetraethylsilanediamineBCP30198SilanediaMide, N,N,N',N'-tetraethylBis(diethylamino)silane, 99% (99.999%-Si) BDEAS PURATREMMolecular FormulaC8H22N2SiMolecular Weight174.359InChIInChI=1S/C8H20N2Si/c1-5-9(6-2)11-10(7-3)8-4/h5-8H2,1-4H3 InChI KeyMVYGKQJKGZHAAI-UHFFFAOYSA-N C8H20N2Si/c1-5-9(6-2)11-10(7-3)8-4/h5-8H2,1-4H3 Isomeric SMILESCCN(CC)N(CC)CC
Physical Data
AppearanceTransparent liquid
Boiling Point, °CPressure (Boiling Point), Torr7015
Spectra
Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Chemical shifts1Hchloroform-d1
Description (IR Spectroscopy)Solvent (IR Spectroscopy)Temperature (IR Spectroscopy), °CBandsgas2971 - 790 cm**(-1)
Route of Synthesis (ROS)
Route of Synthesis (ROS) of Bis(diethylamino)dihydrosilane CAS: 27804-64-4
ConditionsYielddiethylamine With n-butyllithium In hexane at -60℃; for 15h; Inert atmosphere; Large scale;With Dichlorosilane In hexane at -60℃; for 15h; Temperature; Inert atmosphere; Large scale;Experimental ProcedureUnder a nitrogen atmosphere, was added 5000ml of n-butyllithium in n-hexane in the reaction vessel, at a concentration of 2.5 moles per liter. Under stirring, 1000 g of diethylamine was added dropwise to the reaction kettle to control the dropping rate to prevent the temperature of the system from being kept at -60 ° C. After the dropping was completed, the temperature inside the kettle was kept at -60 And the mixture was stirred for 15 hours. Then pass into dichlorosilane 690 grams, control passed into the kettle to maintain a speed of -60 , and finally stirred at -60 after 15 hours, warmed to room temperature. Hexane solvent was distilled off at atmospheric pressure and then vacuum distilled bis (diethylamino) silane, distillation pressure 15mmHg, distillation temperature of 70 . To give product 990 g, yield (with respect to the weight of silicon molar ratio) was 91.1%.91.1%With ethylene bis(tetrahydroindenyl)titanium dichloride; 4-fluoro-benzamidoxime; zinc 2-ethylhexanoate; Dichlorosilane; copper(II) nitrate; lithium tetraphenylborate tris(1,2-dimethoxyethane) In hexane; water at 60 - 120℃; for 5h; Temperature; Reagent/catalyst; Inert atmosphere;89%With Dichlorosilane In hexane at -25℃; for 3h; Inert atmosphere;75%
Safety and Hazards
No data available
Other Data
TransportationUnder the room temperature and away from lightStorageUnder the room temperature and away from lightShelf Life1 yearMarket Price
DruglikenessLipinski rules componentMolecular Weight174.362logP1.88247HBA2HBD0Matching Lipinski Rules4Veber rules componentPolar Surface Area (PSA)6.48Rotatable Bond (RotB)6Matching Veber Rules2
Use PatternBis(diethylamino)dihydrosilane CAS#: 27804-64-4 be used as an interface modifier in ceramics and glass production, improving surface properties.
https://www.chemwhat.com/bisdiethylaminodihydrosilane-cas-27804-64-4/
Comments
Post a Comment