IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
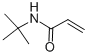
Product NameTert-Butyl AcrylamideIUPAC NameN-tert-butylprop-2-enamide Molecular StructureCAS Registry Number 107-58-4EINECS Number203-505-6MDL NumberMFCD00026271Beilstein Registry Number1742331SynonymsN-TERT-BUTYLACRYLAMIDE107-58-4tert-ButylacrylamideN-(tert-Butyl)acrylamide2-Propenamide, N-(1,1-dimethylethyl)-N-tert-butylprop-2-enamideAcrylamide, N-tert-butyl-N-t-ButylacrylamideNSC 5287EINECS 203-505-6UNII-XJ13FSH48KN-(1,1-Dimethylethyl)-2-propenamideBRN 1742331XJ13FSH48KAI3-25002DTXSID1040114NSC-5287EC 203-505-64-04-00-00664 (Beilstein Handbook Reference)n-tert-butyl acrylamideT-Bu acrylamideMFCD00026271n-tert-butylacrylamidN-t-butyl acrylamideN-tert.-butylacrylamideN-t-Butyl-2-propenamideN- tertiary ButylacrylamideSCHEMBL26705N-tert-butylacrylamide (TBA)N-tert-Butylacrylamide, 97%CHEMBL3184503DTXCID90201142-Propenamide,1-dimethylethyl)-NSC5287N-TERT-BUTYL-2-PROPENAMIDEAMY40862Tox21_301092AKOS005174133CS-W013480NCGC00248285-01NCGC00254992-01AS-17781CAS-107-58-4B0703FT-0675966EN300-64881D70371Q27293858Z440633548InChI=1/C7H13NO/c1-5-6(9)8-7(2,3)4/h5H,1H2,2-4H3,(H,8,9Molecular FormulaC7H13NOMolecular Weight127.184InChIInChI=1S/C7H13NO/c1-5-6(9)8-7(2,3)4/h5H,1H2,2-4H3,(H,8,9)InChI KeyXFHJDMUEHUHAJW-UHFFFAOYSA-N Isomeric SMILESCC(C)(C)NC(=O)C=C
Patent InformationPatent IDTitlePublication DateUS10737259Salt tolerant anion exchange medium2020JP2017/186303MANUFACTURING METHOD OF β-SUBSTITUTED PROPIONIC ACID AMIDE AND N-SUBSTITUTED (METH)ACRYLAMIDE2017 JP2015/209419METHOD OF PRODUCING N-SUBSTITUTED (METH)ACRYLAMIDE2015US2012/231072THERMO-RESPONSIVE HYDROGEL COMPOSITIONS2012US2008/286221Anionic Ethyl Methacrylate Copolymers and Use Thereof2008
Physical Data
AppearanceWhite powder
Melting Point, °C Solvent (Melting Point) 130 - 131hexane124 - 125125 - 127123 - 124129128 - 131water
Density, g·cm-31
Description (Association (MCS))Solvent (Association (MCS))Temperature (Association (MCS)), °CPartner (Association (MCS))Further physical properties of the complex24 - 44N-Isopropylacrylamide, N,N-Dimethylacrylamide
Spectra
Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Temperature (NMR Spectroscopy), °C Frequency (NMR Spectroscopy), MHzChemical shifts, Spectrum1Hchloroform-d1Chemical shifts, Spectrum13Cchloroform-d1Chemical shifts, Spectrum1HCD3ODChemical shifts1Hchloroform-d1500Chemical shifts1Hchloroform-d124.84600
Description (IR Spectroscopy)Solvent (IR Spectroscopy)ATR (attenuated total reflectance), BandsBands, SpectrumBands, Spectrumpotassium bromideBandsnujol
Description (UV/VIS Spectroscopy)Solvent (UV/VIS Spectroscopy)Comment (UV/VIS Spectroscopy)UV/VISSpectrumSpectrum240 - 270 nm
Route of Synthesis (ROS)
Route of Synthesis (ROS) of Tert-Butyl Acrylamide (n-TBAA) CAS 107-58-4
ConditionsYieldWith BF3 immobilized on β-cyclodextrine functionalized silica coated CoFe2O4 magnetic nanoparticles In neat (no solvent) at 20℃; for 0.5h; Ritter Amidation;95%With (2,3,4,5,6-pentafluorophenyl)ammonium triflate; water at 90℃; for 3h; Ritter reaction; Neat (no solvent); chemoselective reaction;92%With sulfuric acid In acetic acid at 20℃; for 2.5h; Ritter reaction; Inert atmosphere; Enzymatic reaction;85%Experimental Procedure General procedure: tert-butyl acetate (2 mmol), nitrile (2.2 mmol), and H2O (2 mmol) were mixed with PFPAT (10 mol %) and heated to 90 °C, until complete disappearance of the starting nitriles (as monitored by TLC). After cooling to room temperature, the organic phase was washed with aqueous 1 M NaOH solution (1 ml). The separated organic phase was evaporated under reduced pressure to give a crude residue, which was purified by distillation or by column chromatography (hexane-EtOAc). The products were characterized by comparison of their physical and spectral data with those of authentic samples. Spectroscopic data for selected examples:
Safety and Hazards
Pictogram(s)SignalWarningGHS Hazard StatementsH302 (100%): Harmful if swallowed H319 (67.37%): Causes serious eye irritation Precautionary Statement CodesP264, P264+P265, P270, P280, P301+P317, P305+P351+P338, P330, P337+P317, and P501(The corresponding statement to each P-code can be found at the GHS Classification page.)
Other Data
TransportationUnder the room temperature and away from lightUnder the room temperature and away from lightHS CodeStorageUnder the room temperature and away from lightShelf Life1 yearMarket Price
DruglikenessLipinski rules componentMolecular Weight127.186logP1.302HBA2HBD1Matching Lipinski Rules4Veber rules componentPolar Surface Area (PSA)29.1Rotatable Bond (RotB)3Matching Veber Rules2
Toxicity/Safety PharmacologyQuantitative Results
Use PatternTert-Butyl Acrylamide (n-TBAA) CAS#: 107-58-4 is white powder. It is a monomer, is used for the production of many polymers and is an intermediate in organic chemical synthesis. (1) Industrial use (2) Paper industries (3) Personal care (4) Thickener. And N-tert-butylacrylamide is an important monomer that can be used in polymerization reactions to prepare high molecular weight polymers.
https://www.chemwhat.com/tert-butyl-acrylamide-n-tbaa-cas-107-58-4/
Comments
Post a Comment