IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
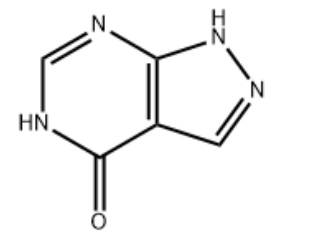
Product NameAllopurinol IUPAC Name1,5-dihydropyrazolopyrimidin-4-oneMolecular StructureCAS Registry Number 315-30-0EINECS Number206-250-9MDL NumberMFCD00599413Synonymsallopurinol315-30-01H-Pyrazolopyrimidin-4-olZyloprimLopurinZyloricSuspendolAtisurilBleminolCaplenalTakanaruminUripurinolEmbarin4-Hydroxypyrazolopyrimidine1H-Pyrazolopyrimidin-4(5H)-oneAllopurinolum1H-Pyrazolo(3,4-d)pyrimidin-4-ol4-Hydroxypyrazolopyrimidine4-Hydroxy-1H-pyrazolo(3,4-d)pyrimidine1H-pyrazolopyrimidin-4(7H)-one4-Hydroxy-3,4-pyrazolopyrimidine4-Hydroxypyrazolo(3,4-d)pyrimidineAlopurinol Allopurinolum 2H-Pyrazolopyrimidin-4-ol1H-pyrazolopyrimidin-4(2H)-one4-Hydroxypyrazolyl(3,4-d)pyrimidine4H-Pyrazolo(3,4-d)pyrimidin-4-one4'-Hydroxypyrazolol(3,4-d)pyrimidine180749-09-11,5-Dihydro-4H-pyrazolo(3,4-d)pyrimidin-4-one1,5-Dihydro-4H-pyrazolopyrimidin-4-one1,5-Dihydro-4H-pyrazolo(3,4-d)pyrimidine-4-one4H-Pyrazolo(3,4-d)pyrimidin-4-one, 1,5-dihydro-4H-Pyrazolopyrimidin-4-one, 1,5-dihydro-1,5-Dihydropyrazolopyrimidin-4-oneMFCD00599413HSDB 3004EINECS 206-250-9UNII-63CZ7GJN5INSC-10165563CZ7GJN5ISMR0000590834-Hydroxy-1H-pyrazolopyrimidineALLOPURINOL SODIUMATH0081H-Pyrazolopyrimidin-4-ol (9CI)4H-Pyrazolopyrimidin-4-one, 1,7-dihydro- (9CI)1H,2H,4H-pyrazolopyrimidin-4-one1H,4H,7H-pyrazolopyrimidin-4-oneDTXSID4022573NSC1390Allopurinol 4H-Pyrazolopyrimidin-4-one, 1,2-dihydro-NSC1016559002-17-9Allopurinol NCGC00015094-02NCGC00094580-044H-Pyrazolopyrimidin-4-one, 2,5-dihydro- (9CI)4H-Pyrazolopyrimidin-4-one, 2,7-dihydro- (9CI)AllohexalBW 56158BW-561581H,4H,5H-pyrazolopyrimidin-4-one1,5-Dihydro-pyrazolopyrimidin-4-oneA 8003DTXCID5025734H-PyrazolopyrimidinePan Quimica4'-HydroxypyrazololpyrimidineCAS-315-30-0SR-05000001983HexanuratUrictoQuimica, PanAllohexal (TN)4-HydroxypyrazolpyrimidinePrestwick_511Xanthomax-100Xanthomax-3004H-Pyrazolopyrimidin-4-one,1,2-dihydro-Zyloric-300Allopurinol (Zyloprim)Spectrum_000026ALLOPURINOL Opera_ID_1680Lopac-A-80031,4-d]pyrimidin-4-oneSCHEMBL4627ALLOPURINOL CHEMBL1467NCIOpen2_001825Lopac0_000102ALLOPURINOL ALLOPURINOL ALLOPURINOL BSPBio_001798KBioGR_000550KBioSS_000386MLS001148183US9138393, AllopurinolUS9144538, AllopurinolDivK1c_000685SPECTRUM1500108SPBio_000056ALLOPURINOLUM GTPL6795SCHEMBL1128219Allopurinol (JP17/USP/INN)BDBM35440HMS502C07KBio1_000685KBio2_000386KBio2_002954KBio2_005522KBio3_001298ALLOPURINOL ALLOPURINOL NINDS_000685ALLOPURINOL BDBM181133HMS1920A15Pharmakon1600-01500108ALLOPURINOL Allopurinol AMY18272BCP26973DRG-0056DUZALLO COMPONENT ALLOPURINOLH-Pyrazolo(3,4-d)pyrimidin-4-olHY-B0219STR05189Tox21_1100824-Hydroxy-pyrazolopyrimidinAC-019BBL009959BDBM50016784BDBM50140241CCG-38916NSC755858s1630SC1118SC2251STK378584STK711106AKOS000267490Tox21_110082_1Allopurinol, xanthine oxidase inhibitorTS-00028SBI-0050090.P0042H-Pyrazolopyrimidin-4-ol (9CI)A0907EU-0100102SW199406-41,5-dihydropyrazolo-pyrimidin-4-oneEN300-34144VU0611037-1BIM-0061756.00014h-pyrazolopyrimidin-4-one,1,7-dihydro-AB-323/25048497Allopurinol (4-Hydroxypyrazolopyrimidine)Q412486SR-010000755951,5-Dihydro-4H-pyrazolo-nupyrimidin-4-one4H-pyrazolopyrimidin-4-one, 1,7-dihydro-J-504736SR-01000075595-1SR-05000001983-1SR-05000001983-2W-1068924H-pirazolo pirimidina-4-ona, 1,5-dihidro-F2173-0394F3329-0375Z104486670Allopurinol, British Pharmacopoeia (BP) Reference StandardAllopurinol, European Pharmacopoeia (EP) Reference StandardAllopurinol, United States Pharmacopeia (USP) Reference Standard1,5-Dihydro-4H-pyrazolopyrimidin-4-one Synonym: AllopurinolAllopurinol, Pharmaceutical Secondary Standard; Certified Reference Material1H-Pyrazolopyrimidin-4-ol;1H-PYRAZOLOPYRIMIDIN-4(5H)-ONE184856-42-6Molecular FormulaC5H4N4OMolecular Weight136.11InChIInChI=1S/C5H4N4O/c10-5-3-1-8-9-4(3)6-2-7-5/h1-2H,(H2,6,7,8,9,10)InChI KeyOFCNXPDARWKPPY-UHFFFAOYSA-NCanonical SMILESC1=NNC2=C1C(=O)NC=N2
Patent InformationPatent IDTitlePublication DateUS2001/53373Process and device for producing liquid dosage formulations of medicinal compounds on demand from tablets and capsules2001
Physical Data
AppearanceOff-white powder
Melting Point, °C 350 - 360350297 - 299
Description (Association (MCS))Solvent (Association (MCS))Temperature (Association (MCS)), °CPartner (Association (MCS))AdsorptionH2O, HCl37cross-linked insoluble polyvinylpyrrolidoneChemisorptionH2O37cross-linked insoluble polyvinylpyrrolidoneChemisorptionH2O, HCl37cross-linked insoluble polyvinylpyrrolidone
Spectra
Description (IR Spectroscopy)Spectrum
Description (UV/VIS Spectroscopy)Solvent (UV/VIS Spectroscopy)Comment (UV/VIS Spectroscopy)Absorption Maxima (UV/VIS), nmSpectrum210 - 330 nmSpectrumH2O200 - 280 nmAbsorption maximaH2O251SpectrumH2O240 - 280 nm
Route of Synthesis (ROS)
Route of Synthesis (ROS) of Allopurinol CAS#: 315-30-0
ConditionsYieldWith N,N-dimethyl-formamide; trichlorophosphate for 12h; Reflux;76%With trichlorophosphate In N,N-dimethyl-aniline for 1h; Reflux;72%With trichlorophosphate In N,N-dimethyl-aniline at 80℃; for 2h;Experimental ProcedureA mixture of allopurinol (2.00 g, 14.69 mmol) and N,N-dimethylaniline (2.00 g, 16.52 mmol) wasstirred in POCl3 (25 mL) at 80 °C for 2 h. The reaction mixture was diluted with water (35 mL), andextracted with ethyl acetate. The organic layer was washed with water and the organic phase wasconcentrated to dryness, and the residue was purified by column chromatography on silica gel using4:1.5 petroleum ether/ethyl acetate as eluent to give 2; white flake solid, 70 % yield.70%
Safety and Hazards
No data available
Other Data
TransportationUnder room temperature away from lightHS CodeStorageUnder room temperature away from lightShelf Life5 yearsMarket Price
DruglikenessLipinski rules componentMolecular Weight136.113logP0.905HBA4HBD2Matching Lipinski Rules4Veber rules componentPolar Surface Area (PSA)74.69Rotatable Bond (RotB)0Matching Veber Rules2
Quantitative Results1 of 800Comment (Pharmacological Data)Bioactivities presentReferenceCompositions suitable for the treatment of damage caused by ischemia/reperfusion or oxidative stress2 of 800Comment (Pharmacological Data)Bioactivities presentReferenceFUSED BICYCLIC NATURAL COMPOUNDS AND THEIR USE AS INHIBITORS OF PARP AND PARP-MEDIATED INFLAMMATORY PROCESSES3 of 800Comment (Pharmacological Data)Bioactivities presentReferencePROTEIN KINASE C ZETA INHIBITION TO TREAT VASCULAR PERMEABILITY
Use PatternAllopurinol CAS#: 315-30-0 is an intermediate in pesticides and dyes; pesticide raw materials; analytical reagents.
https://www.chemwhat.com/allopurinol-cas-315-30-0-2/
Comments
Post a Comment