IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
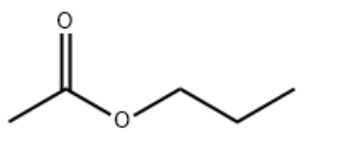
Product NameN-PROPYL ACETATEIUPAC Namepropyl acetate Molecular StructureCAS Registry Number 109-60-4EINECS Number203-686-1MDL NumberMFCD00009372SynonymsPropyl acetate109-60-4N-PROPYL ACETATEAcetic acid, propyl esterPropyl ethanoate1-Acetoxypropanen-Propyl ethanoateOctan propyluAcetic acid n-propyl esterPropylacetateAcetate de propyle normaln-Propyl acetate (natural)Acetic acid propyl esterPropylester kyseliny octoveOctan propylu n-propanol acetateEINECS 203-686-1Acetic acid, n-propyl esterUNII-4AWM8C91G6BRN 17407644AWM8C91G6DTXSID6021901CHEBI:40116AI3-24156Acetate de propyle normal Propylester kyseliny octove DTXCID301901ACETIC ACID,PROPYL ESTEREC 203-686-14-02-00-00138 (Beilstein Handbook Reference)PROPYL ACETATE (USP-RS)n-propylacetatn-Propyl ester of acetic acidacetic acid propylPropyl acetate, N-ACETATE, PROPYLPropyl acetate, 99%PAT (CHRIS Code)Actate de propyle normalCH3COOCH2CH2CH3Propyl ester of acetic acidPROPYL ACETATE FEMA NUMBER 2935SCHEMBL14991PROPYL ACETATE WLN: 3OV1CHEMBL44857PROPYL ACETATE PROPYL ACETATE Propyl acetate, >=99.5%Propyl acetate, >=98%, FGN-PROPYL ACETATE N-Propyl acetate LBG-64752Propyl Acetate (Fragrance Grade)Propyl Acetate (Industrial Grade)ACETIC ACID, N-PROPYL ETHERNSC72025Tox21_202012MFCD00009372NA1276STL280317AKOS008949448Propyl acetate, natural, >=97%, FCC, FGn-Propyl acetate n-Propyl acetate Propyl acetate, United States Pharmacopeia (USP) Reference StandardPropyl Acetate, Pharmaceutical Secondary Standard; Certified Reference MaterialMolecular FormulaC5H10O2Molecular Weight102.13InChIInChI=1S/C5H10O2/c1-3-4-7-5(2)6/h3-4H2,1-2H3InChI KeyYKYONYBAUNKHLG-UHFFFAOYSA-N Canonical SMILESCCCOC(=O)C
Patent InformationPatent IDTitlePublication DateCN107987044A process for manufacturing a δ - e lactone method (by machine translation)2018CN105237342Method for preparing alcohol through catalytic hydrogenation reduction of carboxylate2016WO2014/144685METHODS FOR IDENTIFYING ARTHROPOD REPELLENTS AND ATTRACTANTS, AND COMPOUNDS AND COMPOSITIONS IDENTIFIED BY SUCH METHODS2014US2010/292496Process for Production of Alkyl Tin Alkoxide Compound, and Process for Production of Carbonic Acid Ester Using the Compound2010
Physical Data
AppearanceColorless, clear and no mechanical impurities
Melting Point, °C -95-92.5
Boiling Point, °C101.16101.24100 - 10292101.18
Density, g·cm-3Measurement Temperature, °C0.8599544.990.8769529.990.8656539.990.8713234.99
Description (Association (MCS))Solvent (Association (MCS))Partner (Association (MCS))Sorption diagramRate of adsorption20dinonyl phthalateRate of adsorption20Triton X-305
Spectra
Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Temperature (NMR Spectroscopy), °C Frequency (NMR Spectroscopy), MHzChemical shifts1HChemical shifts, Spectrum1HChemical shifts1Hchloroform-d1600Chemical shifts13Cchloroform-d1Chemical shifts, Spectrum1Hchloroform-d126.84400Chemical shifts, Spectrum13Cchloroform-d126.84100
Description (IR Spectroscopy)Solvent (IR Spectroscopy)Temperature (IR Spectroscopy), °CBands, Spectrum BandsMid IR (MIR), BandsnujolBandsKBrin the presence of organic compounds
Description (UV/VIS Spectroscopy)Solvent (UV/VIS Spectroscopy)Comment (UV/VIS Spectroscopy)Absorption Maxima (UV/VIS), nmExt./Abs. Coefficient, l·mol-1cm-1Absorption maximaH2O, H2SO4Ratio of solvents: 66percent2585740Absorption maximaH2O, NaOHRatio of solvents: 0.1N232, 2908600, 3120
Route of Synthesis (ROS)
Route of Synthesis (ROS) of N-PROPYL ACETATE CAS 109-60-4
ConditionsYieldWith hydrogen In neat (no solvent) at 30℃; for 5h; Catalytic behavior; Flow reactor;100%With hydrogen; 0.5% Pd/C at 80℃; for 100h; Product distribution / selectivity;99.3%With hydrogen; 0.3 % Pd/C In lithium hydroxide monohydrate at 40℃; under 6000.6 Torr; Product distribution / selectivity;99.97%Experimental ProcedureThird distillation process:21 parts by mass of the distillate (Yl) obtained in the second distillation process of Example 1 were supplied to a third distillation column, and distilled under the following conditions: top pressure of 5 kPaG, bottom pressure of 45 kPaG, top temperature of 105°C, bottom temperature of 135°C, reflux ratio of 1.7, and evaporation rate of 96% by mass. A distillate (Zl) containing high-purity allyl acetate was obtained from the top of the distillation column. The concentration of water in the obtained distillate (Zl) was 30 ppm, the concentration of allyl acetate was 99.74% by mass, the concentration of acetic acid was less than 5 ppm, and the concentration of allyl acrylate was less than 5 ppm.Hydrogenation process:Next, the distillate (Zl) was subjected to a hydrogenation reaction in the same manner as in Example 1, thus obtaining n-propyl acetate.With regard to the obtained n-propyl acetate, the conversion rate of allyl acetate was 99.9%, the selectivity of l-propenyl acetate was 0.21%, the selectivity of acetic acid was 0.25%, and the yield of n-propyl acetate was 99.97%. In addition, theconcentration of propyl propionate in the obtained reaction liquid was less than 5 ppm.99.97%
Safety and Hazards
Pictogram(s)SignalDangerGHS Hazard StatementsH225: Highly Flammable liquid and vapor H319: Causes serious eye irritation H336: May cause drowsiness or dizziness Precautionary Statement CodesP210, P233, P240, P241, P242, P243, P261, P264+P265, P271, P280, P303+P361+P353, P304+P340, P305+P351+P338, P319, P337+P317, P370+P378, P403+P233, P403+P235, P405, and P501(The corresponding statement to each P-code can be found at the GHS Classification page.)
Other Data
TransportationUnder room temperature away from lightHS CodeStorageUnder room temperature away from lightShelf Life1 yearMarket Price
DruglikenessLipinski rules componentMolecular Weight102.133logP1.022HBA2HBD0Matching Lipinski Rules4Veber rules componentPolar Surface Area (PSA)26.3Rotatable Bond (RotB)3Matching Veber Rules2
Quantitative Results1 of 203Comment (Pharmacological Data)Bioactivities presentReferencePROCESS FOR PRODUCING HYDROGENATED ESTER, HYDROGENATION CATALYST FOR USE THEREIN, AND PROCESS FOR PRODUCING THE CATALYST2 of 203Comment (Pharmacological Data)Bioactivities presentReferenceHigh affinity ligands for nociceptin receptor ORL-13 of 203Comment (Pharmacological Data)Bioactivities presentReferenceSUBSTITUTED PIPERIDINES AS MELANOCORTIN RECEPTOR AGONISTS4 of 203Comment (Pharmacological Data)Bioactivities presentReferenceAN EFFICIENT PROCESS FOR THE MANUFACTURE OF (E)-ENTACAPONE POLYMORPHIC FORM A5 of 203Comment (Pharmacological Data)Bioactivities presentReferenceTc-labeled arylpiperazine derivatives for imaging serotonin receptor10 of 10Resultseffect on phosphatidylcholine secretion in primary cultures of rat type II pneumocytes
Use PatternN-PROPYL ACETATE CAS#: 109-60-4 is a versatile organic solvent with good solubility and volatility. It is used to dissolve various organic compounds, including resins, coatings, paints, adhesives, and coating materials.
https://www.chemwhat.com/n-propyl-acetate-cas-109-60-4/
Comments
Post a Comment