IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
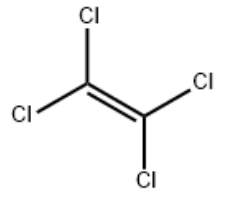
Product NamePERCHLOROETHYLENEIUPAC Name 1,1,2,2-tetrachloroethene Molecular StructureCAS Registry Number 127-18-4EINECS Number204-825-9MDL NumberMFCD00000834SynonymsTETRACHLOROETHYLENETetrachloroethene127-18-4Perchlorethylene1,1,2,2-TetrachloroethyleneEthylene tetrachlorideCarbon dichlorideTetrachloraethenPerchloorethyleen, perEthylene, tetrachloro-PerchloroetheneRcra waste number U210Percloroetilene Perclene TGCzterochloroetylen Tetrachloroethylene (IUPAC)Perchloorethyleen, per EINECS 204-825-9Tetrachloroethylene RCRA waste no. U210UNII-TJ904HH8SNEPA Pesticide Chemical Code 078501BRN 1361721TJ904HH8SNTETRACHLOROETHYLENE-1-13CNSC-9777EC 204-825-94-01-00-00715 (Beilstein Handbook Reference)MFCD0000083425135-99-3Tetrachloroethene 100 microg/mL in MethanolNema (VAN)WLN: GYGUYGGTTE (CHRIS Code)Freon 1110Tetrachlooretheen(DUTCH)Tetrachloraethen(GERMAN)bmse000633Czterochloroetylen(POLISH)1,2,2-TetrachloroethyleneC2-Cl4SCHEMBL23022BIDD:ER03461,1,2,2-tetrachloro-ethenePerchloorethyleen, per(DUTCH)Perchloraethylen, per(GERMAN)Perchlorethylene, per(FRENCH)Perchloroethylene Reagent Grade1,1,2, 2-TetrachloroethyleneTetrachloroethylene, >=99.5%Eteno, 1,1,2,2-tetracloro-TETRACHLOROETHYLENE Tetrachloroethylene, UV/IR-GradeEthene, 1,1,2,2-tetrachloro-TETRACHLOROETHYLENE perchloroethylene (tetrachloroethylene)Tetrachloroethylene, anhydrous, >=99%Tetrachoroethylene; see PerchloroethyleneCAS-127-18-4Perchloroethylene; (Tetrachloroethylene)Tetrachloroethylene Tetrachloroethylene, for HPLC, >=99.9%Tetrachloroethylene, ReagentPlus(R), 99%Tetrachloroethylene, for synthesis, 99.0%Tetrachloroethylene, ACS reagent, >=99.0%EN300-19890Tetrachloroethene 1000 microg/mL in MethanolTetrachloroethene 5000 microg/mL in Methanol1,1,2,2-Tetrachloroethylene (ACD/Name 4.0)Tetrachloroethylene, SAJ first grade, >=98.0%Tetrachloroethylene, SAJ special grade, >=99.0%Tetrachloroethylene, UV HPLC spectroscopic, 99.9%TETRACHLOROETHYLENE (PERCHLOROETHYLENE) Tetrachloroethylene, Ultrapure, Spectrophotometric GradeMolecular FormulaC2Cl4Molecular Weight165.8InChIInChI=1S/C2Cl4/c3-1(4)2(5)6 InChI KeyCYTYCFOTNPOANT-UHFFFAOYSA-N Canonical SMILESC(=C(Cl)Cl)(Cl)Cl
Patent InformationPatent IDTitlePublication DateCN111454122Method for eliminating hydrogen chloride by catalytic cracking of chloralkane2020WO2014/62255METHOD OF PREPARING HALOGENATED SILAHYDROCARBYLENES2014WO2010/45104PYRROLOPYRIDAZINE DERIVATIVES2010US6362383Hydro-fluorination of chlorinated hydrocarbons2002
Physical Data
AppearanceColorless, clear and no mechanical impurities
Melting Point, °C Comment (Melting Point)13.5Mol(s) H2O-22.35-22.18-23.5-19
Boiling Point, °CPressure (Boiling Point), Torr72 - 7316012255150.015121.1121.1121760.051121
Density, g·cm-3Measurement Temperature, °C1.61273261.6227201.6144924.991.6224.841.614824.99
Description (Association (MCS))Solvent (Association (MCS))Temperature (Association (MCS)), °CPartner (Association (MCS))Enthalpy of adsorptiongaseous matrixcalcined ordered mesoporous silica MCM-41Adsorption isothermgaseous matrix86.84calcined ordered mesoporous silica MCM-41Adsorption and desorption isotherms24.84calcined ordered mesoporous silica MCM-41Adsorption and desorption isotherms24.84Cr3+-exchanged natural clinoptilolite from UkraineEnthalpy of adsorptiongaseous matrixCr3+-exchanged natural clinoptilolite from Ukraine
Spectra
Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Frequency (NMR Spectroscopy), MHzChemical shifts13Ctetrahydrofuran-d8Spectrum13CChemical shifts13C75.5CIDNP13C
Description (IR Spectroscopy)Solvent (IR Spectroscopy)Temperature (IR Spectroscopy), °CSpectrumBandssolid matrix-269.16Bandssolid Ar-261.15SpectrumnujolReflection spectrumSpectrum24.85
Description (UV/VIS Spectroscopy)Solvent (UV/VIS Spectroscopy)Comment (UV/VIS Spectroscopy)Absorption Maxima (UV/VIS), nmExt./Abs. Coefficient, l·mol-1cm-1Spectrumhexaneconcentration dependenceSpectrumgas190 - 290 nmAbsorption maximavarious solvent(s)258.515488.1Absorption maximaethyl acetate25915136Absorption maximadioxane259.59550
Route of Synthesis (ROS)
Route of Synthesis (ROS) of PERCHLOROETHYLENE CAS 127-18-4
ConditionsYieldWith hydrogen fluoride; chlorine; antimony pentafluoride at 126 - 148℃; under 17251.7 - 20252 Torr; for 7.1h;A 51.5%B 44%With hydrogen fluoride; antimony pentafluoride at 145 - 148℃; under 19126.9 Torr; for 4.6h;A 27.7%B 39.8%With hydrogen fluoride; antimony pentafluoride at 106 - 147℃; under 16126.6 - 18001.8 Torr; for 5.3h;Experimental ProcedureExample 4 (comparative) In this experiment, the same catalyst and apparatus as the previous experiment were used. A series of reactions were carried out in the autoclave using the same catalyst. Catalyst fluorination : Antimony pentachloride was added to the autoclave and the autoclave was sealed. Anhydrous hydrogen fluoride (192.8g, 9.64m) was then added in aliquots, venting through the scrubber chain, as required. The autoclave was then heated over 4 hours to 117C and allowed to cool. Run 1: Perchloroethylene (59.8g, 0.361m) was charged to the autoclave. The autoclave was then heated to 106C over 2 hours and the pressure rose to 2.4 MPa (23.0 barg). Venting was then commenced through the scrubber chain. The autoclave was then further heated to 141C over 3.3 hours, while the pressure was maintained between 2.15-2.40 MPa (20.5-23.0 barg).A 21.1%B 30.5%
Safety and Hazards
Pictogram(s)SignalWarningGHS Hazard StatementsH351: Suspected of causing cancer H411: Toxic to aquatic life with long lasting effects Precautionary Statement CodesP203, P273, P280, P318, P391, P405, and P501(The corresponding statement to each P-code can be found at the GHS Classification page.)
Other Data
TransportationUnder room temperature away from lightHS CodeStorageUnder room temperature away from lightShelf Life1 yearMarket Price
DruglikenessLipinski rules componentMolecular Weight165.834logP3.026HBA0HBD0Matching Lipinski Rules4Polar Surface Area (PSA)0Rotatable Bond (RotB)0Matching Veber Rules2
Quantitative Results1 of 262Comment (Pharmacological Data)Bioactivities presentReferenceProcess for the preparation of dibenzothiepin derivative2 of 262Comment (Pharmacological Data)Bioactivities presentReference 3 of 262Comment (Pharmacological Data)Bioactivities presentReferenceProcess for the production of polyfunctional cyanic acid esters4 of 262Comment (Pharmacological Data)Bioactivities presentReferencePROCESS FOR PURIFYING PENTAFLUOROETHANE, PROCESS FOR PRODUCING THE SAME, AND USE THEREOF5 of 262Comment (Pharmacological Data)Bioactivities presentReferenceAntifungal paints and coatings
Use PatternPERCHLOROETHYLENE CAS#: 127-18-4 can be usde in metal sur face cleaning,clothes dry cleaning,fossil fuel extracting ,medicine producing ,organic synthesizi ng,and oil,rubber,resin alkaloids,wax dissolving.
https://www.chemwhat.com/perchloroethylene-cas-127-18-4/
Comments
Post a Comment