IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
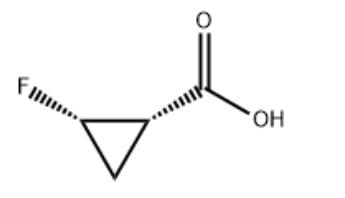
Product Name(1S,2S)-2-Fluorocyclopropanecarboxylic acidIUPAC Name(1S,2S)-2-fluorocyclopropane-1-carboxylic acid Molecular StructureCAS Registry Number 127199-14-8Synonyms(1S,2S)-2-fluorocyclopropanecarboxylic acid127199-14-8105919-34-4cis-2-fluorocyclopropanecarboxylic acid(1s,2s)-2-fluorocyclopropane-1-carboxylic acidcis-2-Fluorocyclopropane-1-carboxylic acidcis-2-Fluoro-cyclopropanecarboxylic acid(1S,2S)-rel-2-Fluorocyclopropanecarboxylic acidCyclopropanecarboxylic acid, 2-fluoro-, (1S-cis)-MFCD19237459(cis)-2-fluorocyclopropanecarboxylic acidCyclopropanecarboxylic acid, 2-fluoro-, (1S,2S)-(1S,2S)-2-fluorocyclopropanecarboxylicacidMFCD04972869SCHEMBL1463730AMY5475DTXSID901262266CFA19914CS1221AKOS006294678Molecular FormulaC4H5FO2Molecular Weight104.08InChIInChI=1S/C4H5FO2/c5-3-1-2(3)4(6)7/h2-3H,1H2,(H,6,7)/t2-,3+/m1/s1 InChI KeyHZQKMZGKYVDMCT-GBXIJSLDSA-NCanonical SMILESC1(1F)C(=O)O
Physical Data
AppearanceWhite solid
Melting Point, °C Solvent (Melting Point) 55 - 5662 - 63toluene
Spectra
Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Frequency (NMR Spectroscopy), MHzChemical shifts1Hchloroform-d6400Chemical shifts, Spectrum1Hchloroform-d1400Chemical shifts, Spectrum1Hchloroform-d1100Chemical shifts1HCDCl3Chemical shifts19FCDCl3
Description (IR Spectroscopy)Solvent (IR Spectroscopy)Temperature (IR Spectroscopy), °CATR (attenuated total reflectance), BandsBandsKBr3210 - 1190 cm**(-1)
Route of Synthesis (ROS)
Route of Synthesis (ROS) of (1S,2S)-2-Fluorocyclopropanecarboxylic acid
ConditionsYieldWith N-ethyl-N,N-diisopropylamine; N-pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In N,N-dimethyl-formamide at 10 - 30℃; for 5h;83.4%With N-ethyl-N,N-diisopropylamine; N-pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In N,N-dimethyl-formamide at 20℃;1.1 gExperimental ProcedureDMF (50 mL), (1S,2S)-2-fluorocyclopropanecarboxylic acid (425 mg, 4.1 mmol), HATU (2.1 g, 5.58 mmol) and DIEA (1.44 g, 11.16 mmol) were successively added to compound 4a (1.0 g, 3.71 mmol), and the mixture was stirred at room temperature for 5 h. The reaction was quenched by adding water, extracted 3 times with ethyl acetate and washed twice with saturated brine. The organic phase was dried and concentrated, and the residue was separated and purified by silica gel column chromatography (eluent: EA/PE=1/2) to obtain 117E (1.1 g, 83.4%). LC-MS (ESI): m/z=356.3 +.
Safety and Hazards
Pictogram(s)SignalDangerGHS Hazard StatementsH314 (100%): Causes severe skin burns and eye damage H335 (100%): May cause respiratory irritation Precautionary Statement CodesP260, P261, P264, P271, P280, P301+P330+P331, P302+P361+P354, P304+P340, P305+P354+P338, P316, P319, P321, P363, P403+P233, P405, and P501(The corresponding statement to each P-code can be found at the GHS Classification page.)
Other Data
TransportationStore in room temperature for long time and dry place; in container tightly sealed; Protect from light.HS CodeStorageStore in room temperature for long time and dry place; in container tightly sealed; Protect from light.Shelf Life2 yearsMarket Price
DruglikenessLipinski rules componentMolecular Weight104.081logP0.27HBA2HBD1Matching Lipinski Rules4Veber rules componentPolar Surface Area (PSA)37.3Rotatable Bond (RotB)1Matching Veber Rules2
Use Pattern(1S,2S)-2-Fluorocyclopropanecarboxylic acid CAS#: 127199-14-8 and its derivatives can act as chiral catalysts in asymmetric synthesis reactions. They can guide the reaction selectively to produce chiral products, thereby enhancing the stereo-selectivity and efficiency of the reaction.
https://www.chemwhat.com/1s2s-2-fluorocyclopropanecarboxylic-acid-cas-127199-14-8-2/
Comments
Post a Comment