IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
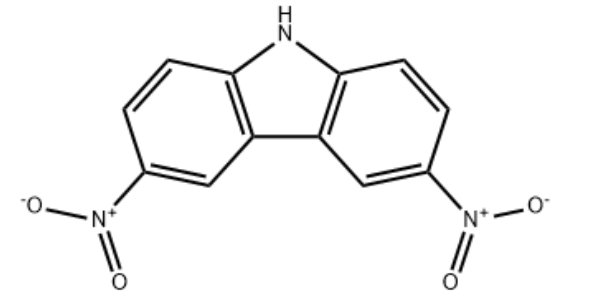
Product Name3,6-Dinitro-9H-carbazoleIUPAC Name3,6-dinitro-9H-carbazoleMolecular StructureCAS Registry Number 3244-54-0EINECS Number221-814-4Synonyms3,6-Dinitro-9H-carbazole3244-54-03,6-DinitrocarbazoleCarbazole, 3,6-dinitro-9H-Carbazole,3,6-dinitro-CCRIS 67729H-Carbazole, 3,6-dinitro-EINECS 221-814-4L5YYP3DVN9C12H7N3O4Maybridge1_002104UNII-L5YYP3DVN9Oprea1_673803SCHEMBL155013HMS547H14DTXSID80186179STK673606AKOS003653972AS-75794LS-188308CS-0094238N1691310.14272/IARXLBTXILYASE-UHFFFAOYSA-N.1EN300-18547434doi:10.14272/IARXLBTXILYASE-UHFFFAOYSA-N.1Q63408793Molecular FormulaC12H7N3O4Molecular Weight257.2InChIInChI=1S/C12H7N3O4/c16-14(17)7-1-3-11-9(5-7)10-6-8(15(18)19)2-4-12(10)13-11/h1-6,13HInChI KeyIARXLBTXILYASE-UHFFFAOYSA-N Canonical SMILESC1=CC2=C(C=C1(=O))C3=C(N2)C=CC(=C3)(=O)
Patent InformationPatent IDTitlePublication Date CN113200906Carbazole triamine derivative and preparation method and application thereof2021US2004/138301Chemical uncouplers for the treatment of obesity2004
Physical Data
Melting Point, °C Solvent (Melting Point) 242 - 243242 - 243300383 - 385dimethylformamide351386 - 387nitrobenzene
Description (Association (MCS))Solvent (Association (MCS))Temperature (Association (MCS)), °CPartner (Association (MCS))Stability constant of the complex with ...acetonitrile26.853,6-diamino-9H-carbazole
Spectra
Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Temperature (NMR Spectroscopy), °C Frequency (NMR Spectroscopy), MHzChemical shifts1Hdimethylsulfoxide-d6400Chemical shifts1Hacetonitrile400Chemical shifts, Spectrum1Hdimethylsulfoxide-d6400Spectrum1Hdimethylsulfoxide-d6300.151
3,6-Dinitro-9H-carbazole CAS#: 3244-54-0 NMR
Description (IR Spectroscopy)Solvent (IR Spectroscopy)Temperature (IR Spectroscopy), °CBandspotassium bromideBandspotassium bromideSpectrumKBrBandsKBr
Description (UV/VIS Spectroscopy)Solvent (UV/VIS Spectroscopy)Comment (UV/VIS Spectroscopy)acetonitrileRemark: 298 KSpectrumacetonitrileRemark: 298 KUV excited state absorptionSpectrumacetonitrile
Route of Synthesis (ROS)
Route of Synthesis (ROS) of 3,6-Dinitro-9H-carbazole CAS 3244-54-0
ConditionsYieldWith copper(II) nitrate monohydrate; acetic anhydride; acetic acid at 100℃; for 0.5h;85%With copper(II) nitrate trihydrate; acetic anhydride; acetic acid at 100℃; for 0.5h;85%With copper nitrate hemi(pentahydrate); acetic anhydride; acetic acid at 15 - 90℃; for 1.16667h;81%Experimental ProcedureA homogeneous mixture of Cu(NO3)2·2.5H2O (30 mmol), acetic acid (20 mL), and acetic anhydride (30 mL) was prepared at room temperature. To this solution were added carbazole (25 mmol) in small portions over 10 min. Temperature was maintained at 15-20 °C during addition of carbazole. The temperature was allowed to rise to room temperature over a period of 30 min and then to 90 °C. Reaction was continued with stirring for a period of 30 min at this temperature. The mixture was poured into 250 mL of distilled water with constant stirring. The precipitate was collected by filtration, and washed five times each with about 100 mL of distilled water. The filtrate was dried in vacuumat. Chromatography on silica gel, eluting with petroleum/EtOAC (3:1) gave 2 (5.23 g, 81%) as a yellow solid. mp >300 °C (lit.27 mp >300 °C). 1H NMR (400 MHz, DMSO-d6) δ 12.69 (s, 1H), 9.50 (d, J = 2.1 Hz, 2H), 8.40 (dd, J = 9.0, 2.1 Hz, 2H), 7.77 (d, J = 9.0 Hz, 2H). ESI-MS m/z: 258+.
Safety and Hazards
No data available
Other Data
TransportationUnder room temperature away from lightUnder room temperature away from lightHS CodeStorageUnder room temperature away from lightShelf Life1 yearMarket Price
DruglikenessLipinski rules componentMolecular Weight257.205logP3.315HBA1HBD1Matching Lipinski Rules4Veber rules componentPolar Surface Area (PSA)107.43Rotatable Bond (RotB)2Matching Veber Rules2
Use Pattern3,6-Dinitro-9H-carbazole CAS#: 3244-54-0 is a compound with fluorescence properties and can be used in the preparation of luminescent materials. It may have potential optoelectronic performance in organic light-emitting diodes (OLEDs) and fluorescent dyes.
https://www.chemwhat.com/36-dinitro-9h-carbazole-cas-3244-54-0-2/
Comments
Post a Comment