IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
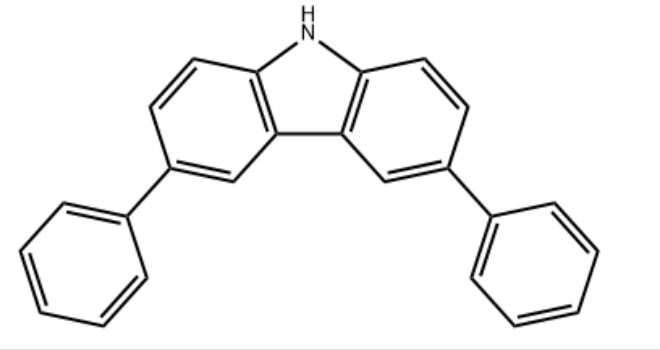
Product Name3,6-Diphenyl-9H-carbazoleIUPAC Name3,6-diphenyl-9H-carbazole Molecular StructureCAS Registry Number 56525-79-2MDL NumberMFCD00222619Synonyms3,6-Diphenyl-9H-carbazole56525-79-23,6-diphenylcarbazole9H-Carbazole, 3,6-diphenyl-MFCD002226196654-68-83,6-diphenyl carbazole3,6-Biphenyl-9H-carbazoleOprea1_545753Oprea1_8152423,6-di-phenyl-9h-carbazoleSCHEMBL1496853,6-Diphenylcarbazole, 99%YSZC1788AMY8728DTXSID80985149STK927806AKOS002336524OL10012SB66927AC-28755AS-10598SY036866CS-0080917D4433FT-0733900A831073Molecular FormulaC24H17NMolecular Weight319.4InChIInChI=1S/C24H17N/c1-3-7-17(8-4-1)19-11-13-23-21(15-19)22-16-20(12-14-24(22)25-23)18-9-5-2-6-10-18/h1-16,25H InChI KeyPCMKGEAHIZDRFL-UHFFFAOYSA-N Canonical SMILESC1=CC=C(C=C1)C2=CC3=C(C=C2)NC4=C3C=C(C=C4)C5=CC=CC=C5
Patent InformationPatent IDTitlePublication DateCN114656396Organic compound and application thereof, and organic electroluminescent device containing organic compound2022KR2021/75622Novel compound and organic light emitting device comprising the same2021KR2021/75621Novel compound and organic light emitting device comprising the same2021KR2021/75620Novel compound and organic light emitting device comprising the same2021CN112876406Deuterated carbazole compound, preparation method thereof, photoelectric material and medicine2021CN113620811Halogenation method of aromatic compound2021
Physical Data
AppearanceOff-white solid
Melting Point, °C Solvent (Melting Point) 177.1186hexane, ethyl acetate186
Spectra
Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Temperature (NMR Spectroscopy), °C Frequency (NMR Spectroscopy), MHzChemical shifts1Hchloroform-d1400Chemical shifts1Hchloroform-d1400Chemical shifts13Cchloroform-d1Chemical shifts, Spectrum1Hchloroform-d1600Chemical shifts, Spectrum13Cchloroform-d1151Chemical shifts, Spectrum1HChemical shifts, Spectrum1H400
Description (IR Spectroscopy)Solvent (IR Spectroscopy)BandsKBr
Description (UV/VIS Spectroscopy)Solvent (UV/VIS Spectroscopy)Absorption Maxima (UV/VIS), nmSpectrumN,N-dimethyl-formamideSpectrumtolueneSpectrumtetrahydrofuran256, 294
Route of Synthesis (ROS)
Route of Synthesis (ROS) of 3,6-Diphenyl-9H-carbazole CAS 56525-79-2
ConditionsYieldWith tetrakis(triphenylphosphine) palladium(0); potassium carbonate In 1,4-dioxane; water at 90℃;86%With tetrakis(triphenylphosphine) palladium(0); potassium carbonate In 1,4-dioxane at 90℃; for 24h; Inert atmosphere;86%With tetrakis(triphenylphosphine) palladium(0); potassium carbonate In ethanol; water; toluene at 80℃; for 20h; Inert atmosphere;83%Experimental Procedure Add 3,6-dibromocarbazole (9.75g, 30.00mmol, 1.0 equivalent) to a dry three-necked flask with a magnetic stirring rotor and condenser.Phenylboronic acid (8.78g, 72.00mmol, 2.4 equivalents),Tetrakis(triphenylphosphine)palladium (347mg, 0.3mmol, 10mol%),Potassium carbonate (20.73g, 150.00mmol, 3 equivalents),Then pump nitrogen three times,Add toluene/ethanol/water (30mL/30mL/10mL) under nitrogen protection.The mixture was stirred and reacted in an oil bath at 80°C for 20 hours.TLC monitors until the reaction of the raw materials is complete, and cool to room temperature.A small amount of water was added and extracted twice with dichloromethane.The organic phases were combined, dried over anhydrous sodium sulfate, and filtered.The solvent was distilled off under reduced pressure.The obtained crude product is separated and purified by silica gel chromatography column, eluent: petroleum ether/ethyl acetate=20:1-5:1,The intermediate 3,6-diphenylcarbazole was obtained, 7.95 g of light brown solid, and the yield was 83%.
Safety and Hazards
Pictogram(s)SignalWarningGHS Hazard StatementsH302 (33.33%): Harmful if swallowed H315 (100%): Causes skin irritation H317 (33.33%): May cause an allergic skin reaction H319 (100%): Causes serious eye irritation H335 (33.33%): May cause respiratory irritation Precautionary Statement CodesP261, P264, P264+P265, P270, P271, P272, P280, P301+P317, P302+P352, P304+P340, P305+P351+P338, P319, P321, P330, P332+P317, P333+P313, P337+P317, P362+P364, P403+P233, P405, and P501(The corresponding statement to each P-code can be found at the GHS Classification page.)
Other Data
TransportationUnder room temperature away from lightUnder room temperature away from lightHS CodeStorageUnder room temperature away from lightShelf Life1 yearMarket Price
DruglikenessLipinski rules componentMolecular Weight319.406logP7.411HBA1HBD1Matching Lipinski Rules3Veber rules componentPolar Surface Area (PSA)15.79Rotatable Bond (RotB)2Matching Veber Rules2
Use Pattern3,6-Diphenyl-9H-carbazole CAS#: 56525-79-2 can be used as a luminescent layer material in organic light-emitting diodes (OLEDs).
https://www.chemwhat.com/36-diphenyl-9h-carbazole-cas-56525-79-2-3/
Comments
Post a Comment