IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
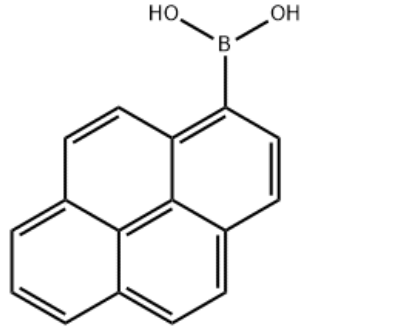
Product Name1-Pyrenylboronic acidIUPAC Namepyren-1-ylboronic acidMolecular StructureCAS Registry Number 164461-18-1MDL NumberMFCD00006400Synonyms1-Pyrenylboronic acidPyren-1-ylboronic Acid164461-18-11-Pyreneboronic acidPyrene-1-boronic acidBoronic acid, 1-pyrenyl-1-pyrenyl boronic acid(pyren-1-yl)boronic acidBoronic acid, B-1-pyrenyl-MFCD049740621-Pyreneboronic acid (contains varying amounts of Anhydride)C16H11BO2pyreneboronic acidpyren-1-boronic acidpyren-1-ylboronicacidSCHEMBL224652YSSJ01086DTXSID60408164BBL103871Pyrene-1-boronic acid, >=95.0%STL557681AKOS015840457AB21450CS-W010239FD14070GS-6482OL10090AM808106SY022635FT-0653019P1625pyren-1-ylboronic acid;1-Pyreneboronic AcidA810568J-010142J-5240831-Pyreneboronic Acid, (contains varying amounts of Anhydride)Molecular FormulaC16H11BO2Molecular Weight246.1InChIInChI=1S/C16H11BO2/c18-17(19)14-9-7-12-5-4-10-2-1-3-11-6-8-13(14)16(12)15(10)11/h1-9,18-19H InChI KeyInChI=1S/C16H11BO2/c18-17(19)14-9-7-12-5-4-10-2-1-3-11-6-8-13(14)16(12)15(10)11/h1-9,18-19H Canonical SMILESB(C1=C2C=CC3=CC=CC4=C3C2=C(C=C1)C=C4)(O)O
Patent InformationPatent IDTitlePublication Date CN111592540Imidazonaphthyridine high-performance photoelectric material and application thereof2020CN109180569Pyrene structure-containing compound, and organic luminescent device thereof2019 CN107652224Compound based on dehydrogenated abietic-acid aromatic ring and preparation method thereof2018CN108822049Bipolar compound based on pyrene-triazole derivative and application thereof2018 CN108947926Bipolar compound based on pyrene-oxadiazole derivative and application of bipolar compound2018
Physical Data
AppearanceYellow powder
Melting Point, °C 293 - 297
Description (Association (MCS))Solvent (Association (MCS))Partner (Association (MCS))Association with compoundaq. buffer, dimethyl sulfoxide3,4-dihydroxyphenylethylamineAssociation with compoundaq. buffer, dimethyl sulfoxideL-epinephrineAssociation with compoundaq. buffer, dimethyl sulfoxidenorepinephrineAssociation with compoundaq. buffer, dimethyl sulfoxide7-diethylaminocoumarine-3-aldehyde, L-epinephrineAssociation with compoundacetonitriletetrabutylammonium sulfate
Spectra
Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Temperature (NMR Spectroscopy), °C Frequency (NMR Spectroscopy), MHzSpectrum11BCD3ODChemical shifts, Spectrum1Hdimethylsulfoxide-d6300Chemical shifts, Spectrum13Cdimethylsulfoxide-d675Chemical shifts1Htetrahydrofuran-d8300Chemical shifts1Hdimethylsulfoxide-d6400Chemical shifts13Cdimethylsulfoxide-d6100
Description (IR Spectroscopy)Solvent (IR Spectroscopy)BandsKBr
Description (UV/VIS Spectroscopy)Solvent (UV/VIS Spectroscopy)SpectrumwaterSpectrumtoluene
Route of Synthesis (ROS)
Route of Synthesis (ROS) of 1-Pyrenylboronic acid CAS164461-18-1
ConditionsYieldWith sodium carbonate; tetrakis(triphenylphosphine) palladium(0) In water; toluene at 80℃; for 8h;79%With tetrakis(triphenylphosphine) palladium(0); potassium carbonate In ethanol; water; toluene at 110℃;76%With tetrakis(triphenylphosphine) palladium(0); potassium carbonate In tetrahydrofuran for 4h; Suzuki Coupling; Reflux;60%Experimental ProcedureTetrakistriphenylphosphine palladium (2.1 g, 1.83 mmol) and potassium carbonate(75.7 g, 549 mmol) added to 1-indole boric acid(46.0 g, 187 mmol) and p-bromoiodobenzene (51.6 g, 183 mmol)a solution in degassed tetrahydrofuran (500 mL),And the mixture was heated under reflux for 4 hours.Hot filtration, a large amount of solid is obtained, and the solid is dissolved in a solvent.After concentration,Compound d1 was obtained by silica gel column chromatography(39.2 g, yield 60%).
Safety and Hazards
Pictogram(s)SignalWarningGHS Hazard StatementsH315 (100%): Causes skin irritation H319 (100%): Causes serious eye irritation H335 (100%): May cause respiratory irritation Precautionary Statement CodesP261, P264, P264+P265, P271, P280, P302+P352, P304+P340, P305+P351+P338, P319, P321, P332+P317, P337+P317, P362+P364, P403+P233, P405, and P501(The corresponding statement to each P-code can be found at the GHS Classification page.)
Other Data
TransportationCool, dry place with tighten contairierHS CodeStorageCool, dry place with tighten contairierShelf Life1 yearMarket Price
DruglikenessLipinski rules componentMolecular Weight246.073logP4.171HBA2HBD2Matching Lipinski Rules4Veber rules componentPolar Surface Area (PSA)40.46Rotatable Bond (RotB)1Matching Veber Rules2
Use Pattern1-Pyrenylboronic acid CAS:#164461-18-1 can form coordination complexes with metal ions through the boronic acid group's coordination ability. These complexes have important applications in catalysis, materials science, and bioanalysis. And 1-Pyreneboronic acid can undergo carbon-carbon bond formation reactions with suitable reactants, such as organic halides or aromatic aldehydes. This reaction is often employed in organic synthesis for carbon-carbon bond connections and the construction of complex organic molecules.
https://www.chemwhat.com/1-pyrenylboronic-acid-cas164461-18-1/
Comments
Post a Comment