IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
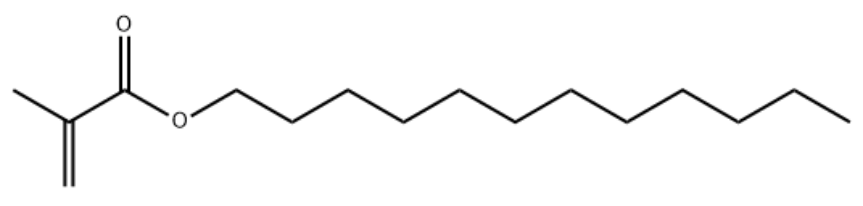
Product NameLauryl Methacrylate (LMA)IUPAC Namedodecyl 2-methylprop-2-enoateMolecular StructureCAS Registry Number 142-90-5EINECS Number205-570-6MDL NumberMFCD00008972SynonymsDodecyl methacrylate142-90-5LAURYL METHACRYLATEDodecyl 2-methylacrylateN-Dodecyl methacrylateMetazene2-Propenoic acid, 2-methyl-, dodecyl esterdodecyl 2-methylprop-2-enoateDodecyl 2-methyl-2-propenoateSipomer LMAMethacrylic acid, dodecyl esterLAMAAgeflex FM 246Methacrylic acid, lauryl esterGE 410 (methacrylate)Laurylester kyseliny methakryloveLaurylmethacrylateNSC 5188SR 313n-Lauryl methacrylateAcrylic acid, 2-methyl-, dodecyl esterDTXSID4027103B6L83074BZC16H30O2NSC-5188Dodecyl methacrylate (stabilized with MEHQ)DTXCID107103Caswell No. 521Dodecyl-2-methylacrylateCAS-142-90-5HSDB 5417EINECS 205-570-6EPA Pesticide Chemical Code 053101BRN 1708160Laurylester kyseliny methakrylove UNII-B6L83074BZAI3-08765Ageflex FM-121-Dodecyl methacrylatemethacrylic acid dodecyl2-Methyl-2-propenoic acid, dodecyl ester1-Dodecanol methacrylateEC 205-570-6Dodecyl 2-methylacrylate #SCHEMBL14995WLN: 12OVYU1Methacrylic Acid Lauryl EsterMethacrylic Acid Dodecyl EsterCHEMBL1903701NSC5188LAURYL METHACRYLATE Tox21_201903Tox21_303316MFCD00008972N-DODECYL METHACRYLATE AKOS015903634CS-W012588Methyl-2-propenoic acid, dodecyl esterLauryl methacrylate(5cp(25 degrees c))NCGC00164408-01NCGC00164408-02NCGC00257059-01NCGC00259452-01170292-57-6AS-76599Propenoic acid, 2-methyl-, dodecyl esterDB-042652Dodecyl ester of 2-methyl-2-propenoic acidFT-0625575M0083Dodecyl Methacrylate, (stabilized with MEHQ)Lauryl methacrylate, purum, >=95.0% (GC)E75856A807982J-007716Q3395664Lauryl methacrylate, contains 500 ppm MEHQ as inhibitor, 96%Molecular FormulaC16H30O2Molecular Weight254.41InChIInChI=1S/C16H30O2/c1-4-5-6-7-8-9-10-11-12-13-14-18-16(17)15(2)3/h2,4-14H2,1,3H3InChI KeyGMSCBRSQMRDRCD-UHFFFAOYSA-N Canonical SMILESCCCCCCCCCCCCOC(=O)C(=C)C
Patent InformationPatent IDTitlePublication DateCN114656358Method for preparing olefin-containing ester compound under catalysis of deep eutectic solvent2022CN114524789Method for synthesizing 3, 3-disubstituted isobenzofuran-1 (3H)-ketone with enantioselectivity2022CN114702517Application of chitosan Schiff base loaded bivalent copper material in preparation of beta-boryl ester 2022CN106831665Method for enantioselective synthesis of gamma-substituted-gamma-butyrolactone and delta-substituted-delta-valerolactone2017JP2016/6032COSMETICS HAVING COPOLYMER2016
Physical Data
AppearanceColorless to yellow liquid
Melting Point, °C -7
Boiling Point, °CPressure (Boiling Point), Torr130 - 14010167101424
Density, g·cm-3Reference Temperature, °CMeasurement Temperature, °C0.874200.8755200.87174250.8735420
Spectra
Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Frequency (NMR Spectroscopy), MHzChemical shifts, Spectrum13Cchloroform-d1101Chemical shifts, Spectrum1Hchloroform-d1400Chemical shifts, Spectrum1Hchloroform-d1400Chemical shifts13Cchloroform-d1100Spectrum1Hchloroform-d1Chemical shifts1Hchloroform-d1400Chemical shifts13Cchloroform-d1100
Description (IR Spectroscopy)Solvent (IR Spectroscopy)Temperature (IR Spectroscopy), °CBandsneat (no solvent) Bands, SpectrumATR (attenuated total reflectance), Bands, Spectrum25
Route of Synthesis (ROS)
Route of Synthesis (ROS) of Lauryl Methacrylate (LMA) CAS142-90-5
ConditionsYieldWith choline chloride; toluene-4-sulfonic acid; hydroquinone at 100℃; for 6h;94%With dicyclohexyl-carbodiimide In dichloromethane at 20℃; for 4h;86%With phosphoric acid; (p-tolueneslfonic acid, sulfosalicylic acid, resin KU-2x8); hydroquinone In tolueneWith dicyclohexyl-carbodiimide In dichloromethane at 20℃; for 4.16667h; Cooling with ice;Experimental ProcedureAccording to the previous reported work, the reduction of lauricacid ester by lithium aluminum hydride (LAH) was done to producethe target lauryl alcohol . In an ice bath system, dropwiseof a solution of lauryl alcohol (10 mmol) dissolved in dry DCM wasadded to a solution of DCC (11 mmol) and methacrylic acid(10 mmol) in DCM (20 mL) with continuous stirring for 10 mins.The reaction was stirred for 4 hrs at room temperature. After this time, the reaction was filtrated to remove dicyclohexyl urea, thefiltrate was concentrated under vacuum, and the LMA productwas purified using silica gel as an adsorbent (eluent: DCM) in columnchromatography to yield lauryl methacrylate .
Safety and Hazards
Pictogram(s)SignalWarningGHS Hazard StatementsH335: May cause respiratory irritation Precautionary Statement CodesP261, P271, P304+P340, P319, P403+P233, P405, and P501(The corresponding statement to each P-code can be found at the GHS Classification page.)
Other Data
TransportationUnder the room temperature and away from lightHS CodeStorageUnder the room temperature and away from lightShelf Life1 yearMarket Price
DruglikenessLipinski rules componentMolecular Weight254.413logP6.616HBA2HBD0Matching Lipinski Rules3Veber rules componentPolar Surface Area (PSA)26.3Rotatable Bond (RotB)13Matching Veber Rules1
Quantitative Results1 of 98Comment (Pharmacological Data)Bioactivities presentReferenceEmulsion and composition comprising a fluorohydrocarbon compound and a method for preparing such an emulsion and composition2 of 98 Comment (Pharmacological Data)Bioactivities presentReferenceSURFACE-ACTIVE n- ALKYL SULFO(METHYL)PROPIONATES3 of 98Comment (Pharmacological Data)Bioactivities presentReference4 of 98Comment (Pharmacological Data)Bioactivities present5 of 98Comment (Pharmacological Data)Bioactivities presentReferenceStability of monomer emulsion droplets and implications for polymerizations therein6 of 98Comment (Pharmacological Data)Bioactivities presentReferenceMacromolecular surfactants for miniemulsion polymerization7 of 98Comment (Pharmacological Data)Bioactivities presentReferencePreparation and nonlinear optical response of novel palladium-containing micellar nanohybrids
Use PatternLauryl Methacrylate (LMA) CAS 142-90-5 is a commonly used surfactant and emulsifier that has found wide application in various fields. Firstly, Lauryl Methacrylate (LMA) CAS 142-90-5 in the personal care and cleaning agents industries, methyl laurate methacrylate is used as an excellent detergent and foaming agent, providing good cleaning performance and foamability. Additionally, it is often used in the production of personal care products such as shampoo, conditioner, body wash, soap.
https://www.chemwhat.com/lauryl-methacrylate-lma-cas-142-90-5/
Comments
Post a Comment