IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
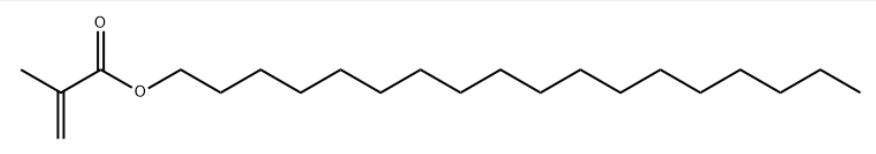
Product NameStearyl methacrylate (SMA)Molecular StructureCAS Registry Number 32360-05-7EINECS Number251-013-5MDL NumberMFCD00026683Synonyms2-Propenoic acid, 2-methyl-, octadecyl ester 32360-05-7 Méthacrylate d'octadécyle MFCD00026683 N-Octadecyl methacrylateoctadecyl 2-methyl-2-propenoateOctadecyl 2-methylacrylateoctadecyl 2-methylprop-2-enoateOctadecyl methacrylate Octadecylmethacrylat POLY(OCTADECYL ACRYLATE)Stearyl methacrylate 112-08-3 167633-23-0 2-Methyl-2-propenoic acid octadecyl ester2-methylacrylic acid stearyl ester2-methylprop-2-enoic acid octadecyl ester55778-34-2 59471-20-4 EINECS 251-013-5Methacrylic Acid Octadecyl Estermethacrylic acid stearyl esterMethacrylic acid, octadecyl esterMethacrylic acid, stearyl esterOctadecyl methacrylate (stabilised with MEHQ)Octadecyl methacrylate contains 90 - 150 ppm MEHQ as inhibitorOctadecyl methacrylate, stabilized with MEHQOctadecylmethacrylateST5409840Stearyl Methacrylate (stabilized with MEHQ)STEARYLMETHACRYLATEMolecular FormulaC22H42O2Molecular Weight338.568InChIInChI=1S/C22H42O2/c1-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20-24-22(23)21(2)3/h2,4-20H2,1,3H3InChI KeyHMZGPNHSPWNGEP-UHFFFAOYSA-NCanonical SMILESOctadecyl methacrylate
Patent InformationPatent IDTitlePublication DateJP2016/6032COSMETICS HAVING COPOLYMER2016 US2011/143622FLUORINATED ACRYLATE COMPOSITIONS2011US2008/175809Silicone copolymer and cosmetics comprising the same2008US2008/199416Use of Anionically and Cationically Ampholytic Copolymers2008US5154920Disinfectant polymeric coatings for hard surfaces1992
Physical Data
AppearanceColorless to pale yellow liquid (@30℃)
Melting Point, °C 21.118 - 2017
Boiling Point, °CPressure (Boiling Point), Torr235101750.51956
Density, g·cm-3Reference Temperature, °CMeasurement Temperature, °C1.144251.24-1901.24
Description (Association (MCS))Solvent (Association (MCS))Temperature (Association (MCS)), °CPartner (Association (MCS))Pressure-surface isothermCHCl321.85H2O
Spectra
Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Temperature (NMR Spectroscopy), °C Frequency (NMR Spectroscopy), MHzChemical shifts1Hchloroform-d125500Chemical shifts13Cchloroform-d125125Chemical shifts1Hchloroform-d1300
Description (IR Spectroscopy)Solvent (IR Spectroscopy)Temperature (IR Spectroscopy), °CBands, Spectrumneat liquid15Bands, Spectrumneat (no solvent, solid phase)15Bands, Spectrum
Route of Synthesis (ROS)
Route of Synthesis (ROS) of Stearyl methacrylate (SMA) CAS 32360-05-7
ConditionsYieldWith 10H-phenothiazine; hydroquinone at 110 - 130℃; for 4.5h; Reagent/catalyst;Experimental ProcedureFirst, 270 parts by mass of stearyl alcohol is added to the reaction vessel, the temperature is appropriately raised to melt it completely, and then 1 part by mass of hydroquinone, 0.3 parts by mass of phenothiazine, and 64.5 parts by mass of methacrylic acid (alkyd mole Ratio 1:0.75), after stirring evenly, add 1.5 parts by mass of sulfonic acid type acrylic cation exchange resin with 8% cross-linking degree, heat the mixture to 110°C, keep refluxing for 2h, and separate the water, then reduce pressure Continue to react for 0.5h. Add 43 parts by mass of methacrylic acid (alkyd molar ratio 1:0.5), and increase the temperature to 130°C for 2h,And separate the water, and then continue the reaction under reduced pressure for 1 hour, and then steam out the remaining water and excess acrylic acid. Then the temperature was lowered to 105°C, and the strong acid cation exchange resin was separated by filtration. The filtrate is then alkali washed. The process is as follows: first add 40 parts by mass of water, then add 20 parts by mass of saturated sodium chloride solution, and finally add 15 parts by mass of sodium carbonate lye with a concentration of 15%, stir for 30 minutes and then stand still Liquid separation. After alkaline washing, the product is washed with water to neutrality, and then decolorized and dried at 65°C.A colorless and transparent stearyl methacrylate was obtained.96%In toluene at 110 - 120℃;Experimental ProcedureGeneral procedure: The initial monomers used in the production of PPDs were alkylmethacrylate (RMC), VA, and N-phenylmethylpropionamide(NPM). Fig. 1 shows their synthetic routines. The RMC monomerswere synthesized by esterification reaction. Methacrylic acid andn-alkyl alcohol at a molar ratio of 1:1.2 were dissolved in toluene,and the reaction was performed at 110 C-120 C for 4 h. The catalystwas p-toluene sulfonic acid (0.1 % by weight), and the polymerizationinhibitor was hydroquinone (0.05 % by weight). Theobtained esterified product was washed with an aqueous NaOHsolution, and finally washed with distilled water to neutrality. Inaddition, the NPM monomers were prepared by acylation reactionof aniline and methacryloyl chloride at a molar ratio of 1.2:1. Thesolvent (N, N-dimethylformamide) and acid binding agent (anhydrouspotassium carbonate) were added and reacted at room temperaturefor 2 h.
Safety and Hazards
No data available
Other Data
TransportationStored in a cool, dry place, away from direct sunlight. HS CodeStorageStored in a cool, dry place, away from direct sunlight. Shelf Life1 yearMarket Price
DruglikenessLipinski rules componentMolecular Weight338.574logP10.03HBA2HBD0Matching Lipinski Rules3Veber rules componentPolar Surface Area (PSA)26.3Rotatable Bond (RotB)19Matching Veber Rules1
Quantitative Results1 of 74Comment (Pharmacological Data)Bioactivities presentReferenceAzo monomer useful in polymerization systems2 of 74Comment (Pharmacological Data)Bioactivities presentReferenceViscosity measurement of a photopolymerized monolayer of a linear-chain polymer3 of 74Comment (Pharmacological Data)Bioactivities presentReferenceStimuli-responsive amphiphilic (co)polymers via RAFT polymerization4 of 74Comment (Pharmacological Data)Bioactivities presentReferenceImproved stability of liposome in oil/water emulsion by association of amphiphilic polymer with liposome and its effect on bioactive skin permeation5 of 74Comment (Pharmacological Data)Bioactivities presentReferenceAnalyses of non-steroidal anti-inflammatory drugs by on-line concentration capillary electrochromatography using poly(stearyl methacrylate-divinylbenzene) monolithic columns6 of 74Comment (Pharmacological Data)Bioactivities presentReferencePEGylation of fluoridated hydroxyapatite (FAp):Ln3+ nanorods for cell imaging
Use PatternStearyl methacrylate (SMA) CAS#:32360-05-7 is known as octadecyl methacrylate and commonly used chemical with a wide range of applications.
https://www.chemwhat.com/stearyl-methacrylate-sma-cas%ef%bc%9a32360-05-7/
Comments
Post a Comment