IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
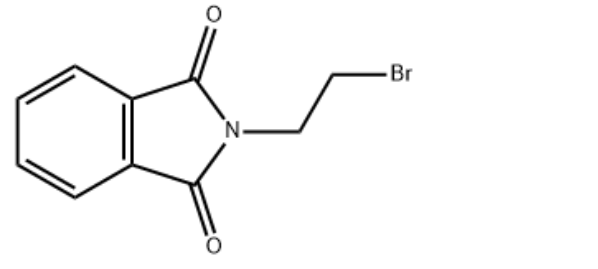
Product NameN-(2-Bromoethyl)phthalimideIUPAC Name2-(2-bromoethyl)isoindole-1,3-dione Molecular StructureCAS Registry Number 574-98-1EINECS Number209-379-9MDL NumberMFCD00005902Beilstein Registry Number148736SynonymsN-(2-BROMOETHYL)PHTHALIMIDE574-98-12-(2-bromoethyl)isoindoline-1,3-dione1H-Isoindole-1,3(2H)-dione, 2-(2-bromoethyl)-2-(2-Bromoethyl)-1H-isoindole-1,3(2H)-dione2-(2-bromoethyl)isoindole-1,3-dione2-(Bromoethyl)phthalimide1-Bromo-2-phthalimidoethanebeta-Phthalimidoethyl bromideMFCD00005902Phthalimide, N-(2-bromoethyl)-NSC 26882-Phthalimidoethyl bromiden(2-bromoethyl)phthalimide.beta.-Bromoethylphthalimide2-(2-Bromo-ethyl)-isoindole-1,3-dioneN-(2-bromoethyl) phthalimide.beta.-Phthalimidoethyl bromideN-(2-Bromoethyl-d4)phthalimide2-(2-bromoethyl)-2,3-dihydro-1H-isoindole-1,3-dione2-(2-bromoethyl)-isoindole-1,3-dionebeta-Bromoethylphthalimide2-(2-Bromoethyl)-1H-isoindole-1,3(2H)-dione; 1-Bromo-2-phthalimidoethane; 2-(1,3-Dioxo-1,3-dihydro-isoindol-2-yl)-ethyl bromide; 2-(2-Bromoethyl)-2,3-dihydro-1H-isoindole-1,3-dioneN-2-BromoethylphthalimideN-(2-Bromoethyl)-phthalimideEINECS 209-379-9N-Bromoethylphthalimidephthalimidoethyl bromide2-(2-bromoethyl)-1H-isoindole-1,3-dioneN-(bromoethyl)phthalimide2-(2-bromoethyl)benzoazoline-1,3-dioneN-(bromoethyl)-phthalimiden(2-bromoethyl) phthalimideN-(2-Bromoethyl)phthalimidSCHEMBL532872-(2-Bromoethyl)phthalimideN-beta-Bromoethyl-phthalimideN-(2-Bromoethyl)-phthalimidN-(2- bromoethyl)phthalimideN-(2-bromo-ethyl)phthalimideN-(Beta-bromoethyl)phthalimideCHEMBL595355N-(2-bromo ethyl)-phthalimideN-(2-bromo-ethyl)-phthalimideN-phthalimideDTXSID0060357NSC2688N-(.beta.-Bromoethyl)phthalimideALBB-017759NSC-2688N-(2-Bromoethyl)phthalimide, 95%AC-165STK291118AKOS0001196991H-Isoindole-1, 2-(2-bromoethyl)-CS-W0089102-(2-Bromo-ethyl)isoindole-1,3-dione2-(2-bromoethyl) isoindoline-1,3-dioneAM808252AS-11033BP-13404SY002569DB-002815B0597FT-0629110EN300-178941H-Isoindole-1,3(2H)-dione,2-(2-bromoethyl)-A831470AE-641/007910322-(2-Bromoethyl)-1H-isoindole-1,3(2H)-dione #Q-201420Z57069240F3380-0002Molecular FormulaC10H8BrNO2Molecular Weight254.08InChIInChI=1S/C10H8BrNO2/c11-5-6-12-9(13)7-3-1-2-4-8(7)10(12)14/h1-4H,5-6H2InChI KeyCHZXTOCAICMPQR-UHFFFAOYSA-N Canonical SMILESC1=CC(=CN=C1)N
Patent InformationPatent IDTitlePublication Date CN104447498A phthalimide derivative and its preparation method and application2017 CN103373951Lapatinib process for the preparation of intermediates2016US2013/195879OXADIAZOLE INHIBITORS OF LEUKOTRIENE PRODUCTION FOR COMBINATION THERAPY2013EP1741709Heteroaryl-substituted amides comprising a saturated linker group, and their use as pharmaceuticals2007
Physical Data
AppearanceOff white crystalline powder
Melting Point, °C Solvent (Melting Point) 83ethanol80 - 8381 - 8485151.3 - 152.580 - 83
Boiling Point, °CPressure (Boiling Point), Torr167617881861020820318
Spectra
Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Frequency (NMR Spectroscopy), MHzChemical shifts, Spectrum1Hchloroform-d1500Chemical shifts13Cchloroform-d1126Chemical shifts, Spectrum1Hdimethylsulfoxide-d6300Chemical shifts, Spectrum13Cdimethylsulfoxide-d675Chemical shifts1Hchloroform-d1400Chemical shifts13Cchloroform-d1100
N-(2-Bromoethyl)phthalimide CAS#:574-98-1
Description (IR Spectroscopy)Solvent (IR Spectroscopy)Temperature (IR Spectroscopy), °CBandspotassium bromide Reflection spectrum, BandsIntensity of IR bands, Bands, Spectrumpotassium bromidein KBrBandsKBr
Description (UV/VIS Spectroscopy)Solvent (UV/VIS Spectroscopy)Comment (UV/VIS Spectroscopy)Spectrumaq. bufferSpectrumCHCl3245 - 340 nm
Route of Synthesis (ROS)
Route of Synthesis (ROS) of N-(2-Bromoethyl)phthalimide CAS 574-98-1
ConditionsYieldWith sodium azide; potassium iodide In water; acetone at 20℃; for 60h; Inert atmosphere;92%With sodium azide In N,N-dimethyl-formamide at 20℃; for 12h;91%With sodium azide In N,N-dimethyl-formamide for 18h; Inert atmosphere; Reflux;90%Experimental Procedure A mixture of 2.6 g of N-(2-bromoethyl)phthalimide (3,10.2 mmol), 0.87 g of NaN3 (13.4 mmol), in 30 mL of dry DMF was refluxed for 18 h under argon. The mixture was evaporated to dryness; the residue was dissolved in 50 mL of dichloromethane and washed subsequently with 2×50 mL of water, 50 mL of brine, dried over MgSO4, filtered, concentrated and dried under vacuum to give pure compound 5 (2.12 g, 90%) as pearly white solid.1H NMR (CDCl3, 300 MHz, 25 °C) δ=7.85 (m, 2H, Ar-H), 7.70 (m, 2H, Ar-H), 3.88 (t, J=6 Hz, 2H, CH2), 3.56 (t, J=6 Hz, 2H, CH2).13C NMR (CDCl3, 75 MHz, 25 °C) δ=168.4 (ArC), 134.6 (ArCH), 132.2 (ArC), 123.8 (ArCH), 49.3 (CH2), 37.2 (CH2).
Safety and Hazards
No data available
Other Data
TransportationStore in room temperature for long time; Protect from light.Store in room temperature for long time; Protect from light.HS CodeStorageStore in room temperature for long time; Protect from light.Shelf Life1 yearMarket Price
DruglikenessLipinski rules componentMolecular Weight254.083logP2.237HBA3HBD0Matching Lipinski Rules4Veber rules componentPolar Surface Area (PSA)37.38Rotatable Bond (RotB)2Matching Veber Rules2
BioactivityIn vitro: EfficacyQuantitative Results
Quantitative Results1 of 79Comment (Pharmacological Data)Bioactivities presentReferenceNOVEL ISOQUINOLINE DERIVATIVES OR SALTS THEREOF2 of 79Comment (Pharmacological Data)Bioactivities presentReferenceFUROISOQUINOLINE DERIVATIVES, PROCESS FOR PRODUCING THE SAME AND USE THEREOF3 of 79Comment (Pharmacological Data)Bioactivities presentReferenceDELTA-AMINO-GAMMA-HYDROXY-OMEGA-ARYL-ALKANOIC ACID AMIDES4 of 79Comment (Pharmacological Data)Bioactivities presentReferenceNovel thiourea derivatives and the pharmaceutical compositions containing the same5 of 79Comment (Pharmacological Data)Bioactivities presentReferenceINSULIN DERIVATIVES CONJUGATED WITH STRUCTURALLY WELL DEFINED BRANCHED POLYMERS6 of 79Comment (Pharmacological Data)Bioactivities presentReference7 of 79Biological materialBioactivities presentReference8 of 79Comment (Pharmacological Data)Bioactivities presentReference9 of 79Comment (Pharmacological Data)Bioactivities presentReference10 of 79Comment (Pharmacological Data)Bioactivities present
Use PatternN-(2-Bromoethyl)phthalimide CAS#:574-98-1 is used in Pharmaceutical Intermediates,N-Substituted Maleimides, Succinimides
https://www.chemwhat.com/n-2-bromoethylphthalimide-cas%ef%bc%9a574-98-1/
Comments
Post a Comment