IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
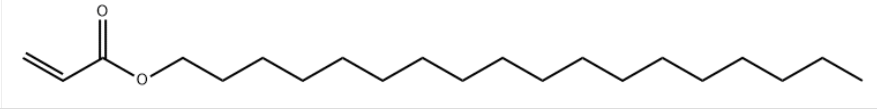
Product NameStearyl acrylate IUPAC Nameoctadecyl prop-2-enoate Molecular StructureCAS Registry Number 4813-57-4EINECS Number225-383-3MDL NumberMFCD00015091SynonymsOctadecyl acrylate4813-57-4Stearyl acrylateoctadecyl prop-2-enoate2-Propenoic acid, octadecyl estern-Octadecyl acrylateAcrylic acid stearyl esterL37WJG7RTHoctadecylacrylateOctadecyl 2-propenoateN-Octadecyl acrylate(11.5 cp(38 degrees c))n-octadecylacrylateEINECS 225-383-3Acrylic acid octadecylVISCOAT STABLEMMER SAEXCEPARL SAI3-15687UNII-L37WJG7RTHLIGHT ACRYLATE S-AEC 225-383-3Acrylic Acid Octadecyl EsterSCHEMBL14967Acrylic acid, octadecyl esterOctadecyl acrylate,high purity1-OCTADECANOL, ACRYLATEDTXSID9063613MFCD00015091Octadecyl acrylate (Stearyl acrylate)AKOS015915480Stearyl Acrylate (stabilized with MEHQ)BS-42313Stearyl Acrylate, (stabilized with MEHQ)A1011CS-0167362FT-0651874D78461A827477Octadecyl acrylate, contains 200 ppm monomethyl ether hydroquinone as inhibitor, 97%Molecular FormulaC21H40O2Molecular Weight324.5InChIInChI=1S/C21H40O2/c1-3-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-20-23-21(22)4-2/h4H,2-3,5-20H2,1H3InChI KeyFSAJWMJJORKPKS-UHFFFAOYSA-N
Patent InformationPatent IDTitlePublication DateCN111116631Organic silicon inhibitor for drilling fluid, and preparation method thereof2020US6492539Preparation of preparing substituted indanones2002
Physical Data
AppearanceWhite waxy solid under room temperature;Colorless to light yellowish liquid under high temperature
Melting Point, °C Solvent (Melting Point) 31 - 3231 - 32.1acetone
Description (Association (MCS))Solvent (Association (MCS))Temperature (Association (MCS)), °CPartner (Association (MCS))Association with compoundH2O25octanol
Spectra
Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Frequency (NMR Spectroscopy), MHzSpectrum1HChemical shifts, Spectrum1HChemical shifts, Spectrum13CChemical shifts1HCD3ODChemical shifts1Hchloroform-d1300
Description (IR Spectroscopy)Solvent (IR Spectroscopy)Temperature (IR Spectroscopy), °CSpectrumBands, Spectrumpotassium bromide14.85 - 54.85Bands, SpectrumBands, Spectrum
Route of Synthesis (ROS)
Route of Synthesis (ROS) of Stearyl acrylate (SA) CAS 4813-57-4
ConditionsYieldWith 10H-phenothiazine; hydroquinone at 110 - 130℃; for 4.5h; Temperature;95.7%With 10H-phenothiazine at 254℃; under 20252 Torr; for 0.0119444h; Microwave Irradiation; Autoclave; Industry scale;93%With hydroquinone In cyclohexane at 60 - 140℃; for 6hExperimental Procedure 100 g of octadecanol, 0.8 g of hydroquinone, and 5 g of in situ acid resin were added to the round bottom flask.Stir and heat to 60 ° C, all of which is dissolved in hydroquinone.Add 40 mL of cyclohexane, add 32.89 g of acrylic acid, and warm to 120 ° C.The reaction was carried out for two hours, and then the temperature was raised to 140 ° C for four hours.The reaction is over. The resin was removed by filtration, and the filtrate was analyzed by high performance liquid chromatography.Its conversion rate is 90%. The filtrate is washed with water and neutralized with sodium carbonate solution.Washed to neutrality, and then dried to obtain octadecyl acrylate.The infrared spectrum of the product is shown in Figure 4 as the 4 spectrum.The nuclear magnetic resonance spectrum of the product is shown in Figure 8.The nuclear magnetic resonance carbon spectrum of the product is shown in Fig. 9.
Safety and Hazards
Pictogram(s)SignalWarningGHS Hazard StatementsH315 (100%): Causes skin irritation H317 (53.66%): May cause an allergic skin reaction H319 (99.7%): Causes serious eye irritation H335 (100%): May cause respiratory irritation H411 (99.39%): Toxic to aquatic life with long lasting effects Precautionary Statement CodesP261, P264, P264+P265, P271, P272, P273, P280, P302+P352, P304+P340, P305+P351+P338, P319, P321, P332+P317, P333+P313, P337+P317, P362+P364, P391, P403+P233, P405, and P501(The corresponding statement to each P-code can be found at the GHS Classification page.)
Other Data
Transportationstored in a cool, dry place, away from direct sunlight. The containers should be kept sealed and if opened, should be resealed tightly.As this product tends to crystallize / become wax like, it might be necessary to preheat the product before using. Avoid direct exposure to the eyes and skin.HS CodeStoragestored in a cool, dry place, away from direct sunlight. The containers should be kept sealed and if opened, should be resealed tightly.As this product tends to crystallize / become wax like, it might be necessary to preheat the product before using. Avoid direct exposure to the eyes and skin.Shelf Life1 yearMarket Price
DruglikenessLipinski rules componentMolecular Weight324.547logP9.849HBA2HBD0Matching Lipinski Rules3Veber rules componentPolar Surface Area (PSA)26.3Rotatable Bond (RotB)19Matching Veber Rules1
Use PatternStearyl acrylate (SA) CAS 4813-57-4 is a versatile chemical that finds wide applications in various industries. One of its primary uses is in the manufacture of adhesives and coatings. Octadecyl acrylate serves as a binder in the formulation of pressure-sensitive adhesives and is also used as a reactive diluent in the production of radiation-curable coatings. In addition, it can act as a co-monomer in the synthesis of various polymers, including block copolymers, graft copolymers, and crosslinked polymers.
https://www.chemwhat.com/stearyl-acrylate-sa-cas-4813-57-4/
Comments
Post a Comment