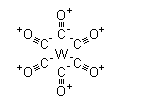
IdentificationPhysical DataSpectraRoute of Synthesis (ROS)Safety and HazardsOther Data
Identification
Product NameTungsten hexacarbonylIUPAC Namecarbon monoxide;tungstenMolecular StructureCAS Registry Number 14040-11-0EINECS Number237-880-2MDL NumberMFCD00011462Beilstein Registry NumberNo data availableSynonymstungsten hexacarbonyl, hexa-carbonyltungstate(0), hexacarbonyl tungsten(0), tungsten(0) hexacarbonyl, hexacarbonyltungsten(0), hexacarbonyltungsten(O), hexacarbonyl tungsten;CAS Number: 14040-11-0;CAS No.: 14040-11-0Molecular FormulaC6O6WMolecular Weight351.9InChIInChI=1S/6CO.W/c6*1-2;InChI KeyFQNHWXHRAUXLFU-UHFFFAOYSA-NCanonical SMILES#.#.#.#.#.#.
Patent InformationPatent IDTitlePublication DateUS2016/24119ELECTRONIC DEVICE COMPRISING METAL COMPLEXES2016EP2733148Tungsten complexes with di-dentate ligands2014
Physical Data
AppearanceWhite crystalline powderSolubilityinsolubleFlash Point200°CSensitivity4: no reaction with water under neutral conditions
Density, g·cm-3Measurement Temperature, °CType (Density)2.6519.84crystallographic
Description (Association (MCS))Association with compound
Spectra
Description (NMR Spectroscopy)Nucleus (NMR Spectroscopy)Solvents (NMR Spectroscopy)Temperature (NMR Spectroscopy), °C Spectrum13Cbenzene-d6Chemical shifts13Cbenzene-d621.8413Cchloroform-d12513Cnot givenSpectrum13C(2)H8-toluene-30Linewidth of NMR absorption13CCDCl330Linewidth of NMR absorption17OCDCl321Spectrum183Wsolid
Description (IR Spectroscopy)Solvent (IR Spectroscopy)Temperature (IR Spectroscopy), °CSignals, cm-1ATR (attenuated total reflectance), BandsBandshexaneIntensity of IR bands, Bandspotassium bromideBandshexane1983BandsKBr1977Bandspotassium bromide1982Bandsgas1.84 - 151.84Bandspolyethylene-83.161980.3
Description (Mass Spectrometry)Comment (Mass Spectrometry)electron impact (EI), spectrumSpectrumFragmentation patternSpectrumMolecular peak, Fragmentation patternFragmentation patternMolecular peakMolecular peak, Fragmentation pattern
Description (UV/VIS Spectroscopy)Solvent (UV/VIS Spectroscopy)Comment (UV/VIS Spectroscopy)Absorption Maxima (UV/VIS), nmSpectrumhexane195 nm - 500 nmSpectrumtetrahydrofuran300 nm - 450 nmSpectrum, Band assignmenthexane375 nm - 720 nmBand assignmentmethylene chloride=methylene dichloride226 nm - 286 nmSpectrum, Band assignmentcyclohexane100 nm - 355 nmBand assignmentmethylene chloride=methylene dichloride288.6Spectrum, Band assignmentmethyl cyclohexane300 nm - 600 nmSpectrum, Band assignmentacetonitrile212.766 nm - 322.581 nm
Description (Raman Spectroscopy)BandsRaman
Tungsten hexacarbonyl CAS#: 14040-11-0 IRTungsten hexacarbonyl CAS#: 14040-11-0 XRD
Route of Synthesis (ROS)
Route of Synthesis (ROS) of Tungsten hexacarbonyl CAS 14040-11-0
ConditionsYieldWith trimethylamine-N-oxide In methanol; benzene byproducts: CO; addn. of soln. of TMNO in methanol to soln. of W(CO)6 in benzene, stirring, then addn. of bipy, stirring, 18h, 28°C, evapn., filtration; recrystn. from CH2Cl2/hexane; elem. anal.;97%In hexane Irradiation (UV/VIS); under N2, excess 2,2'-bipyridine, photolysis with a 200 W Hg lamp; pptn. washed with n-hexane, column chromy. on alumina, recrystn. (CH2Cl2/hexane);80%With sodium tetrahydroborate In butan-1-ol at 105℃; under 760.051 Torr; for 0.333333h; Microwave irradiation; Inert atmosphere; Green chemistry;Experimental ProcedureGeneral Preparation of Mo(CO)4dppe, 2cGeneral procedure: The reactions were run in a CEM Corp. MARS microwave fitted with a fiber optic temperature probe and a port on top for a reflux condenser.1 mmol of Mo(CO)6 and dppe (0.425 g, 1.1 mmol) were combined with 20 mL of 1-propanol in a two-neck 100 mL RB flask. To this mixture was added NaBH4 (0.128 g, 3.3 mmol). The flaskwas placed in themicrowave and a reflux condenser attached through a hole in the top of the microwave. The mixture was sparged with nitrogen. The mixture was heated under nitrogen at 400 W for 1.5 min to reach reflux temperature. Once the reflux temperature was reached the microwave power was reduced and the temperature maintained for 18 min. The mixture was cooled to room temperature and 2-4 mL of water was added to the reaction to dissolve excess NaBH4 and promote product precipitation. The reaction was cooled -10 °C for several hours. The light yellow complex was filtered in air and washed with 2×5 mL of petroleum ether/diethyl ether(1:1) mixture resulting in 580 mg of Mo(CO)4dppe after drying, a 95% yield.80%
Safety and Hazards
GHS Hazard StatementsNot ClassifiedSDS DownloadEnglish Version
Other Data
TransportationClass 6.1; Packaging Group: III; UN Number: 3466Under the room temperature and away from lightHS Code293100StorageUnder the room temperature and away from lightShelf Life1 yearMarket PriceUSD
DruglikenessLipinski rules componentMolecular Weight351.912HBA6HBD0Matching Lipinski Rules3Veber rules componentPolar Surface Area (PSA)0Rotatable Bond (RotB)0Matching Veber Rules2
Use PatternChemical processes/laboratory usecatalyst in method of making a 2-(2,3-epoxypropyl)phenolContrast agent in diagnostic imagingContrast agent in X-ray imaging of atherosclerotic plaqueContrast agent in X-ray imaging of liver tumorsX-ray contrast agentContrast agent in vivo imaging of kupffer cells
Purity CalculationTungsten hexacarbonyl partially dissolves in mixed acids and cannot be directly determined by ICP for the tungsten content in the product. Therefore, impurities in tungsten hexacarbonyl are first dissolved out by mixed acids, and the impurity content in the pure tungsten hexacarbonyl is tested by ICP. The purity of tungsten hexacarbonyl is obtained using the impurity reduction method.The theoretical content of tungsten in tungsten hexacarbonyl is52.23%
https://www.chemwhat.com/tungsten-hexacarbonyl-cas-14040-11-0/
Comments
Post a Comment